Hong Kong J Psychiatry 2002;12(1):6-10
ORIGINAL ARTICLE
S Basu, D Ram, SC Das, SC Gupta
Dr S Basu, MBBS, DPM, Resident, Central Institute of Psychiatry, Kanke, Ranchi, 834006, India.
Dr D Ram, MBBS, MD, Professor of Psychiatry, Central Institute of Psychiatry, Kanke, Ranchi, 834006, India.
Dr SC Das, MBBS, DPM, Resident, Central Institute of Psychiatry, Kanke, Ranchi, 834006, India.
Dr SC Gupta, MBBS, DPM, Resident, Central Institute of Psychiatry, Kanke, Ranchi, 834006, India.
Address for correspondence: Dr D Ram, Professor of Psychiatry, Central Institute of Psychiatry, Kanke, Ranchi, 834006, India.
E-mail: cipranchi@hotmail.com
Submitted: 11 July 2001; Accepted: 26 October 2001
Abstract
Objective:Occurrence of neurological soft signs has not been well researched in childhood and adolescent affective disorder. This study was performed to provide data on the prevalence of neurological soft signs in childhood and adolescent mania, the correlation with disease severity, and relationship to treatment and clinical improvement.
Methods:Thirty four patients (mean age, 16.62 years; SD ± 1.49 years) admitted to hospital with bipolar-I disorder, according to the DSM-IV criteria, were evaluated with the Cambridge Neurological Inventory to assess neurological soft signs and the Young Mania Rating Scale to assess the severity of manic symptoms during the first day of admission. The examination was repeated after 4 weeks. Age- and sex-matched healthy controls were examined using the Cambridge Neurological Inventory.
Results:A significantly high incidence of neurological soft signs was noted in the patient group during both the assessments. However, there was a significant reduction of the total neurological soft signs score (p > 0.001) between the first and the second assessment.
Conclusions:Reduction of neurological soft signs with clinical improvement implies that neurological soft signs is a non-specific marker of neurological abnormality occurring during the height of a mania episode.
Keywords: Neurological soft signs, Childhood, Adolescent, Affective disorder
Introduction
The term ‘neurological soft signs’ (NSS) refers to any neuro- logical deviation — motor, sensory, or integrative — that does not localise the site of a putative central nervous system (CNS) lesion.1 The designation ‘soft’ is usually taken to indicate that the person with the sign shows no other features of fixed or transient neurological lesions or disorders. The clinical importance of soft signs is their value as an indicator of some CNS factors that may have causal or predictive value for associated psychological dysfunction, in particular learning and/or psychiatric abnormalities.2
Various neurological signs are described in a number of psychiatric disorders, including minimal brain dysfunctions,3 emotionally unstable character disorders,1 polysubstance abuse,4 obsessive compulsive disorder,5 persistent emotional disorder in children,6 and consistently so in schizophrenia.7,8 Nasrallah reported that the soft signs are as common in mania as in schizophrenia.9 Mukherjee also reported NSS in bipolar disorder, which was related to the duration of neuroleptic drug exposure.10 Cherian also found a high incidence of NSS in patients with bipolar disorder.11
The presence of NSS in affective disorder is poorly studied and controversial. Most of the trials are single contact stud- ies examining patients when they are symptomatic. Although there has been evidence of a high incidence of NSS in other childhood and adolescent psychiatric disorders, there is a dearth of research into its occurrence in childhood and adolescence mood disorder. The present study aimed to provide data on the prevalence of NSS in childhood and adolescent mania, its correlation with disease severity, and the relation to treatment and clinical improvement.
Methods
Patients
Thirty four patients with mania admitted to the Child Psychiatry Unit of the Central Institute of Psychiatry, Ranchi, diagnosed with bipolar-I disorder, (single manic episode, most recent episode manic) as per the DSM-IV criteria were included in the study. Children and adolescents, aged from 8 to 18 years, of both sexes, cooperated with the study, from 1 November 1998 to 31 October 1999. The exclusion criteria were presence of serious medical illness such as epilepsy, head injury, stroke, diabetes and endocrinological disorders; history of substance abuse defined by DSM IV criteria; mental retardation; pervasive developmental disorder; schizophrenia; pregnancy at entry to the study; or patients undergoing electroconvulsive therapy. The control group con- sisted of age-, sex-, and education- matched individuals from the area, with the same exclusion criteria as the patient group.
Measures
After the initial diagnosis, each patient was further assessed using a structured interview of the Comprehensive Assessment of Symptoms and History (CASH).12 The severity of manic symptoms was assessed using Young’s Mania Rating Scale (YMRS).13
NSS were assessed using the Cambridge Neurological Inventory (CNI),14 a standardised clinical examination with 4 categories of soft signs: motor coordination, sensory integra- tion, primitive reflexes, and failure to suppress inappropriate response. This inventory also assesses hard neurological signs, tardive dyskinesia, catatonic signs, and extrapyramidal signs. However, only soft signs were assessed in this study.
Ratings on the CNI are standardised to indicate ‘normal response’ (0), ‘equivocal response’ (0.5), ‘abnormal response’ (1), or ‘grossly abnormal response’ (2). CNI examination and rating was performed by 2 of the examiners on the initial 15 subjects, each examiner being blind to the examination and the rating of the other examiner. Inter-rater reliability between the examiners was assessed; the k value was found to be 0.85 for the whole scale, indicating high inter-rater reliability. The patient group was assessed on the first day of admission, the second assessment was repeated after 4 weeks. The control group were only assessed once using the CNI.
Eighteen soft signs were evaluated, grouped into the 4 categories already mentioned. ‘Primitive reflexes’ included snout reflex, grasp reflex, and palmo-mental reflex, ‘motor coordination’ included finger-nose test, finger-thumb tapping, finger-thumb opposition, mirror movement for finger-thumb opposition, diadochokinesis, mirror movement for diadocho- kinesis, fist-edge-palm test, and Oseretsky test, ‘failure to suppress inappropriate response’ included rhythm-tapping test and go-no-go test, ‘sensory integration’ included ex- tinction, finger-agnosia, stereognosis, graphaesthesia, and left-right orientation.
Statistical Analysis
Sociodemographic variables were assessed using the descrip- tive statistics. The mean difference in CNI score between the patient and control groups was assessed using the independent samples t-test. The difference between YMRS and CNI scores during the first and second assessment was computed using the t-test for the paired sample. Spearman rank correlation coefficient was determined to examine the associations between the continuous variables.
Results
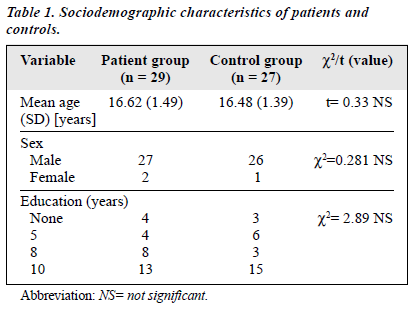
Twenty nine of 34 patients (85.28%) completed the study and there were 27 people in the control group. Twenty seven patients and 26 controls were male. The mean age of the patients was 16.62 years (SD, ± 1.49 years); the mean age of the control group was 16.48 years. The control group was matched with the patient group for educational status (Table 1). The mean duration of illness during the first contact was 2.24 months (SD, ± 2.38 months).
During the first assessment, 89% of the patients were drug-free, 6.89% were receiving antipsychotic agents and lithium and 3.44% were taking antipsychotic agents and carbamazepine. During the second assessment, 55.17% of patients were receiving antipsychotic drugs and lithium, 17.24 % were receiving antipsychotic drugs and carbamazepine, 10.34% were taking antipsychotic agents and sodium valproate, 6.89% were taking only antipsychotic agents, 6.89% were taking antipsychotic agents, lithium, and carbamazepine, and 3.44% were taking antipsychotic agents, lithium, and sodium valproate.
During the first assessment, the mean of the total YMRS score was 38.20 (SD, ± 8.04); during the second assess- ment, this score was reduced to 8.98 (SD, ± 8.43). This reduction in score was highly significant (t value = 14.07; p < 0.001).
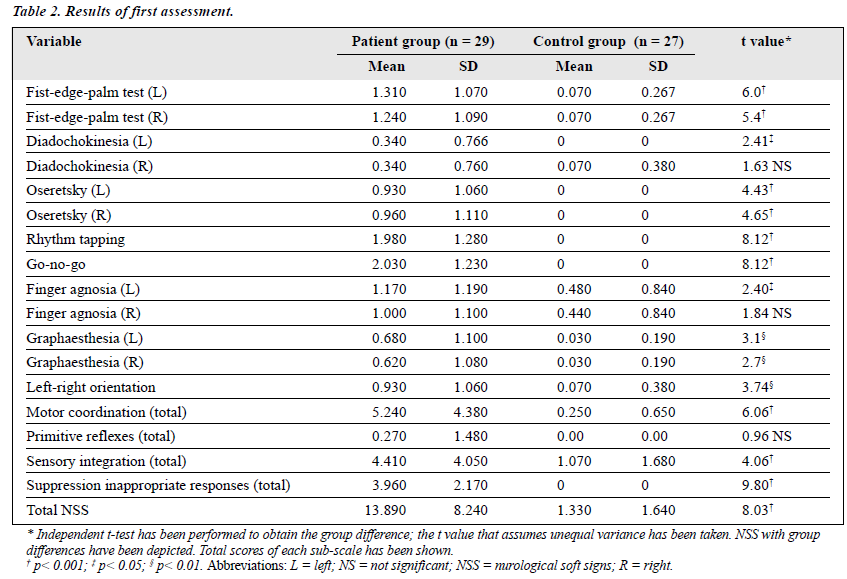
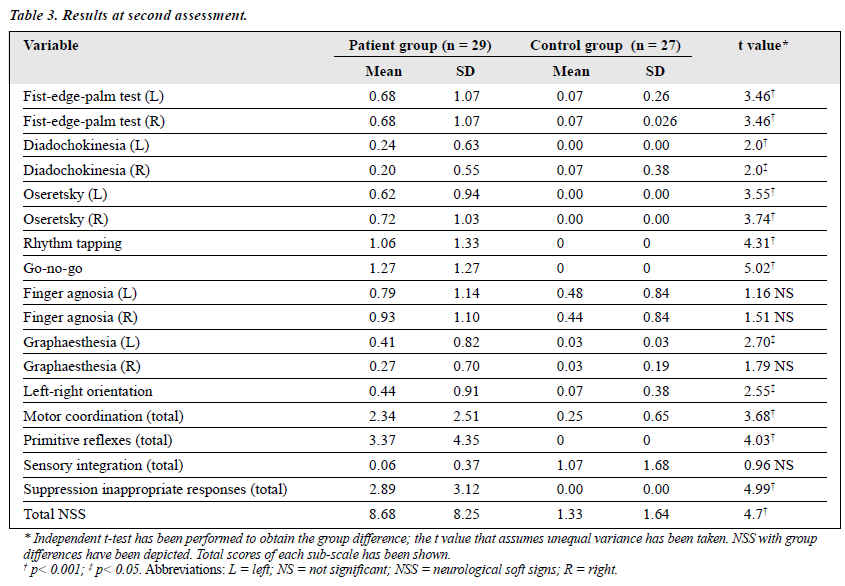
NSS assessment using the CNI showed a significant difference between patients and the controls during both the first and the second assessments. However, the difference was less pronounced during follow-up as compared with the initial assessment (Tables 2 and 3). Among patients, comparison of the first and the second assessments showed a significant reduction at the second assessment. This reduction was significant in the majority of the subtests, including the sens- ory integration (t value = 2.78; p < 0.01), failure to suppress inappropriate responses (t value = 4.04; p < 0.001), and motor coordination (t value = 2.81; p < 0.01). However, there was no significant difference between the first and second assessments in the primitive reflex subtests (Table 4). There was a significant difference between the first and second assessments in total score of NSS (t-value = 4.28; p < 0.001).
Discussion
The findings of this study indicate that NSS are prevalent in symptomatic children and adolescents with mania who have not been taking medication and in the same patients when receiving medication, although with a significant reduction of symptoms. This study thus replicates the findings of previous studies.9-11,15 However, all the previous studies were single contact trials where assessment was performed only at the height of symptoms. In this study, the same group was followed up for a period of 4 weeks. During both the assess- ments, the NSS score or the CNI for patients was significantly higher than for the control group during the symptomatic phase, and the occurrence of NSS was significantly higher during the symptomatic period compared with the asymptomatic period. The total NSS score had a significant positive correla- tion with the total YMRS score, implying that occurrence of NSS was significantly influenced by the symptomatology of mania. This finding replicates the findings of previous studies by Gureji15 who proposed that occurrence of NSS was indicative of neurodysfunction with psychotic state.
While analysing the subgroups of patients with NSS, it became clear that the performances of the rhythm tapping test and the go-no-go test was impaired for most of the patients and, during the second assessment, improvement was most significant in these items. Rhythm tapping and go-no-go tests are measures of attention, concentration, and alertness. These functions are known to be impaired during the height of a manic episode.16 The total score for motor coordination had a significant positive correlation with the total score for failure to suppress inappropriate responses and these items also influenced the total NSS score. Unlike the results of Mukherjee,10 individuals receiving more anti- psychotic agents and mood stabilisers had fewer NSS than when they had symptoms but were drug-free. The use of antipsychotic agents influencing the incidence of NSS has also been noted in patients with schizophrenia17. However, the paradoxical finding in our study can be explained as follows.
Firstly, none of the studies assessed the NSS in the same group of patients when they were both drug-free and receiving antipsychotic agents. Secondly, the improvement in clinical status, which leads to improvement of neuropsychological status, can result in apparent improvement of NSS scores as measured by the CNI.
Previous studies have shown a high incidence of NSS in patients with mania10,15 but our findings are different. In this study, a marked difference was noted between the symptom- atic and the relatively asymptomatic phase, similar to a study of attention deficit and hyperactivity disorder patients managed with stimulants.18 Although the finding in this study implies an apparent improvement of NSS during the follow- up period, even during that period, the NSS scores were significantly higher than in the control group. This finding can be explained by incomplete resolution of symptoms during the follow-up period and also by the high incidence of anti- psychotic use, which is known to influence NSS scores.10
The major limitation of this study is that the examining physician was not blinded to the diagnosis or medication status, resulting in possible clinician bias in neurological evaluation of the patients. Other possible limitations were difficulties in attention and understanding of the instructions during the symptomatic phase.
Efforts were made to overcome this problem by con- ducting the examination after the patient had been made comfortable and by repeating the instructions when needed. The follow-up period was only for 4 weeks duration (in line with the average stay of the patients at the Child Psychiatric Unit). Hence, the majority of the patients remained sympto- matic during this period. The state versus trait controversy with regard to NSS in children and adolescents with mania remains unaddressed. Lastly, the study group of 29 patients was small.
In conclusion, this study suggests that NSS as measured by CNI depends on the severity of the symptoms. Medi- cation paradoxically improved the total NSS score but state versus trait-related issues remained largely unanswered. Further studies with larger groups of patients with a longer duration of follow up are warranted to get a clearer picture of neurological impairment in children and adolescents with mania.
References
- Quitkin F, Rifkin A, Klein DF. Neurological soft signs in schizophre- nia and character disorders. Arch Gen Psychiatry 1976;33:845-853.
- Shaffer D, Schonfeld IS, O’Connor P, et al. Neurological soft signs and their relationship to psychiatric disorder and intelligence in childhood and adolescence. Arch Gen Psychiatry 1985;42:342-351.
- Wilker A, Dixon J, Parker J. Brain function in problem children and controls. Am J Psychiatry 1970;127:94-105.
- Grant IM, Miller M, Reital RW. A neurological study of polydrug Arch Gen Psychiatry 1976;33:973-978.
- Hollander E, Schiffman E, Cohen B, et al. Signs of central nervous system dysfunction in obsessive compulsive disorder. Arch Gen Psychiatry 1990;47;27-32.
- Pine D, Shaffer D, Schenfeld IS. Persistent emotional disorder in chil- dren neurological soft signs. J Am Acad Child Adolesc Psychiatry 1993;32:1229-1236.
- Griffiths TD, Sigmundsson T, Takei N, Rowe D, Murray RM. Neuro- logical abnormalities in familial and sporadic schizophrenia. Brain 1998;121:191-203.
- Chen EY, Lam LC, Chen RY, Nguyen DG, Kwok CL, Au JW. Neuro- logical signs and sustained attention impairment in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2001;251:1-5.
- Nasrallah HA, Tippin J, McCally WM. Neurological soft signs in manic patients. A comparison with schizophrenic and control groups. J Affect Disord 1983;5:45-50.
- Mukherjee S, Shukla S, Rosen A. Neurological abnormalities in patients with bipolar disorder. Biol Psychiatry 1984;19:337-345.
- Cherian A, Kuruvilla K. Prevalence of neurological ‘soft signs’ in affective disorder and their correlation with response to treatment. Indian J Psychiatry 1989;31:224-229.
- Andreasen NC, editor. Comprehensive assessment of symptom and history(CASH). Iowa: University of Iowa; 1987.
- Young RC, Biggs JT, Ziegler VE , Mayer DA. A rating scale for mania reliability validity and sensitivity. Br J Psychiatry 1978;133:429-435.
- Chen EYH, Shapleske J, Luque R, et al. The Cambridge neurological inventory: a clinical instrument for soft neurological signs and the further neurological examination of psychiatric patients. Psychiatr Res 1995;56:183-204.
- Gureji O. Neurological soft signs in Nigerian schizophrenics: a con- trolled study. Acta Psychiatr Scand 1988;78:505-509.
- Braden W, Ho CK. Racing thoughts in psychiatric inpatients. Arch Gen Psychiatry 1981;38:71-75.
- Gupta S, Andreasen NC, Arndt S, et al. Neurological soft signs in neuroleptic-naïve and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry 1995;152:191-196.
- Lerer RJ, Lerer MP. The effects of methylphenidate on the soft neuro- logical signs of hyperactive children. Pediatrics 1976;57:521-525.