Hong Kong J Psychiatry 2006;16:137-43
Original Article
Prevalence of Depression in Chinese People with Parkinson's Disease
華裔帕金遜患者的抑鬱症發病率
DML Chan, WK Tang, V Mok, M Au Yeung, HM Yeung, KS Wong, GS Ungvari
陳蔓蕾、鄧偉光、莫仲棠、歐陽敏、楊漢明、黃家星、GS Ungvari
Dr Doris ML Chan, MBChB, MRCPsych, FHKCPsych, Department of Psychiatry, Shatin Hospital, Hong Kong, China.
Prof WK Tang, MD, Department of Psychiatry, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Dr Vincent Mok, MD, Associate Professor, Department of Medicine & Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Dr M Au Yeung, MBChB, MRCP, FHKCP, FHKAM (Medicine)
Dr Jonas HM Yeung, MBChB, MRCP (UK), FRCP (Edin), FHKCP, FHKAM (Medicine), Department of Medicine, Alice Ho Miu Ling Nethersole Hospital, Tai Po, Hong Kong, China.
Prof KS Wong, MD, Department of Medicine & Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Prof GS Ungvari, PhD, FRANZCP, FRCPsych, FHKCPsych, Department of Psychiatry, The Chinese University of Hong Kong, Shatin, Hong Kong, China.
Tel: (852) 2636 7754; Fax: (852) 2647 5321;
E-mail: dorischan.hk@gmail.com
Submitted: 30 December 2006; Accepted: 13 February 2007
Abstract
Objective: To examine the point prevalence of depressive disorders and their clinical correlates in Chinese patients with Parkinson's disease.
Patients and Methods:Chinese people with Parkinson's disease attending 2 general neurology outpatient clinics were recruited consecutively and a convenience sample was taken from among those attending a Parkinson's disease clinic. The Chinese-Bilingual Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (4th Edition) was used to identify patients who suffered from different kinds of depressive disorder. The demographic and clinical variables of those with depression were compared with those participants who did not have depression.
Results:The point prevalence of any type of depressive disorder was 26.3%. Major depressive episodes were diagnosed in 19 (14.3%) patients, 7 of whom had concurrent dysthymia. Three percent had minor depression and 9% had dysthymia. A history of depression, the age of onset of Parkinson's disease, and global disability were found to be the clinical correlates of depression in Parkinson's disease.
Conclusion:This study indicates that depression is frequent among Chinese people with Parkinson's disease attending neurology clinics in Hong Kong. The clinical correlates of Parkinson's disease found in this study concurred with those found in other studies.
Key words: Parkinson disease; Depression; Prevalence
摘要
目的:探討抑鬱症的點發病率及其與華裔帕金遜患者的臨床相互關係。
患者與方法:訪談連續系列的一組參加兩種神經病學門診的華裔帕金遜患者及另一組方便取樣的帕金遜門診病人。根據中英雙語結構臨床採訪的情緒疾病診斷手冊量表去鑑定病人所患不同類型的抑鬱症。比較參與的抑鬱症患者及無抑鬱症患者不同背景資料和臨床因素。
結果:所有抑鬱症類型的點發病率為26.3%。19(14 .3%) 位被診斷為重抑鬱發作,其中7位競發性心情惡劣。3%病人有輕度抑鬱症,9%病人心情惡劣。帕金遜患者的抑鬱與病人有抑鬱症病史、帕金遜症的發病年齡和機能退化有臨床關係。
結論:這項研究顯示抑鬱在香港神經病學診所的華裔帕金遜患者中普遍可見。研究所得有關帕的臨床因素和其他研究相類似。
關鍵詞:帕金遜病、抑鬱、發病率
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that involves disturbances in motor control. It is a disabling and long-term disease that causes considerable difficulties in the lives of sufferers and their families. The societal burden of PD treatment is expected to escalate, as the population in
Hong Kong is ageing, whilst the mean age of onset of PD is relatively early, at around 60 years.1 In a cross-sectional survey of the residential homes for the elderly in Hong Kong, 3.4% of the subjects were found to have PD.2
It has been shown that a PD patient is most susceptible to depression during the first half year of diagnosis.3 This phenomenon is likely to be a psychological reaction to being labelled as having PD, which is a chronic disease with deterioration in various functional aspects that is not, at present, curable. Younger patients find it more difficult to accept a diagnosis of PD and are more likely to have disruptions in their career, family, and financial aspects.4
Therefore, they are more susceptible to depression. Stress is a known precipitating factor for depression, especially for those who have a history of depression.
Nonetheless, as PD disease progresses, the ‘psychological’ theory alone cannot explain the high prevalence of depression in those with advanced disease. Depression is not only found to precede the onset of PD, but is a risk factor for PD.5 The ‘biological’ theory proposes that depression shares a common neurodegenerative pathway with PD, which makes depression more frequent in PD than expected. Some authors proposed several candidate pathological mechanisms besides dopamine depletion that can cause behavioural symptom, including dysfunction within noradrenergic,6 serotonergic,7 and cholinergic8 systems and neural loss from Lewy bodies and plaques and tangles.9 However, it seems that both ‘psychological’ and ‘biological’ theories interact in complex ways in different PD patients.
Preliminary studies have suggested that depression in PD (dPD) is highly prevalent in the Chinese community. Clinical correlates of dPD are still to be determined. As measurement of the prevalence of dPD is important for resource allocation, comprehensive and methodologically sound studies on this subject are clearly warranted. This study focuses on the frequency of dPD, and its socio- demographic and clinical correlates.
Patients and Methods
Using a cross-sectional design, the data were collected between 1 July 2005 and 30 June 2006. Subjects were identified as having PD by their attending neurologists using the United Kingdom Parkinson ’s Disease Society Brain Bank’s (UKPDSBB10) clinical diagnostic criteria. Those who were of Chinese descent, fluent in the Cantonese dialect, and of any age, were recruited but of these, any who scored 14 or less on the Cantonese-Chinese version of the Mini Mental State Examination (CC-MMSE11) were excluded. People with PD of other aetiologies and a history of stroke, head injury, epilepsy, or other diseases of the central nervous system were excluded.
All the PD patients were identified in 3 clinics. Two were neurology clinics at the Prince of Wales Hospital (PWH) and Alice Ho Miu Ling Nethersole Hospital (AHNH), which are tertiary clinics catering to patients with PD and other neurological diseases in their corresponding catchment areas. The third clinic, the PD clinic at the Pamela Youde Nethersole Eastern Hospital (PYNEH) takes care of patients with idiopathic PD and those with Parkinsonian syndromes caused by different aetiologies. At the PWH and AHNH, the PD patients were recruited as they presented consecutively. At the PYNEH, a convenience sample of PD patients was recruited. Home visits were conducted if the above arrangement was not feasible. All missing data were collected as far as possible by home or telephone interview. Caregivers were invited to participate in the interviews to obtain collateral information. Ethical approvals were obtained from institutional review boards.
Assessment Tools
Socio-demographic and clinical data were collected. The 10 most common chronic diseases among elderly Hong Kong Chinese12 were asked about along with thyroid disease, as this is known to have an association with dPD.13 The research assistant, after being trained by the psychiatrists in use of the scales, administered the Lubben Social Network Scale (LSNS14) and the Hoehn and Yahr Scale (HYS).15 The laterality of hemiparkinsonism and akinesia were recorded. Global disability was assessed by the research assistant using the Schwab and England Activities of Daily Living Scale (SEADL).16 The psychiatrist trained to administer Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders–4th Edition (SCID) was blinded to the demographic and clinical variables of the subjects and interviewed all of the participants using the Chinese-Bilingual SCID Axis I, Patient version (CB-SCID- I/P).17
Statistical Analysis
Data were analysed using the Statistical Package for the Social Sciences for Windows version 11.0. The statistical significance level was set at p < 0.05 unless otherwise specified. For the statistical analysis, patients with major depressive episode (MDE), minor depression (MinD) and dysthymia with or without other psychiatric co-morbidities were combined into 1 group, which was called the ‘any type of depression’ group (AD) whilst those without any depressive disorder formed the ‘no-depression’ group (ND). Descriptive statistics were used to characterise the socio- demographic and clinical profiles of the entire sample, then socio-demographic and clinical variables of the AD and ND groups were compared. Categorical variables were analysed using the Chi-square test or Fisher’s exact test in cases with cell counts of less than 5. Continuous variables were analysed using the t test or the Mann-Whitney U test, depending on the distribution of the data. Where significant differences (p < 0.1) were detected between the AD and ND groups, the variables concerned were entered into a binary logistic regression analysis. The binary logistic regression was employed to examine the impact of different variables on the development of AD.
Results
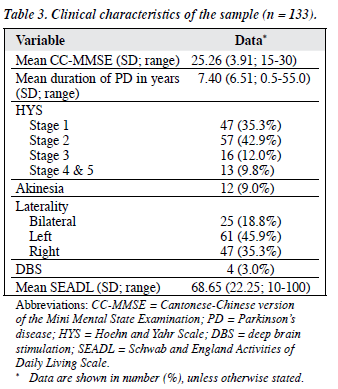
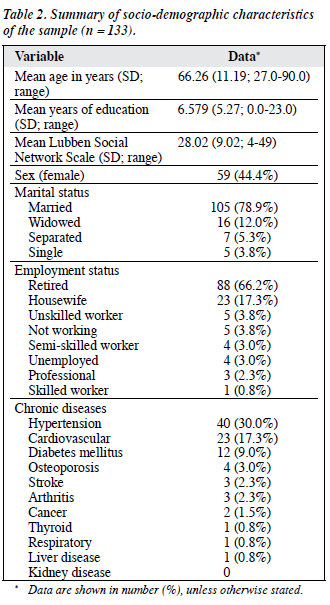
Over the study period, 188 PD patients were recruited. Of eligible subjects, 3 (1.6%) refused to participate and 52 (27.7%) were excluded. Of these, 8 were excluded because of an incomplete data set and 44 because of the exclusion criteria. A total of 133 patients (PWH = 95; AHNH = 13; PYNEH = 25) were finally assessed, giving an overall response rate of 70.7%. Of the 133 patients, 77 (57.9%) had at least 1 caregiver available to provide collateral information. Four patients from the PWH were interviewed at home. There was no statistical difference between the included, excluded, and incomplete data set groups in terms of their sex or the duration of PD (Table 1). Their socio-demographic characteristics are shown in Table 2. Hypertension was the most prevalent chronic disease in the sample population (30.0%) and 23 (17.3%) subjects had cardiovascular diseases. Most patients had milder disease with a large proportion of the patients categorised as being at HYS Stage 2 or below (Table 3).
Prevalence of Depressive Disorders
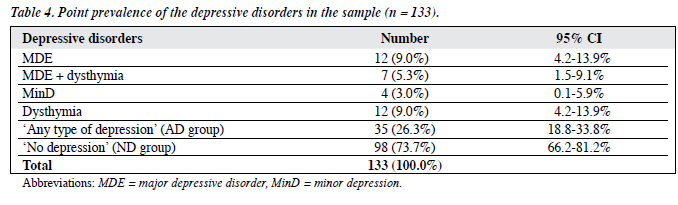
Thirty five (26.3%; 95% CI, 18.8-33.8%) subjects were suffering from some type of depressive disorder. Nineteen (14.3%) subjects were suffering from an MDE; 7 (36.8%) had concurrent dysthymia (Table 4).
Comparison of Characteristics between ‘Any Type of Depression’ and ‘No Depression’ Groups
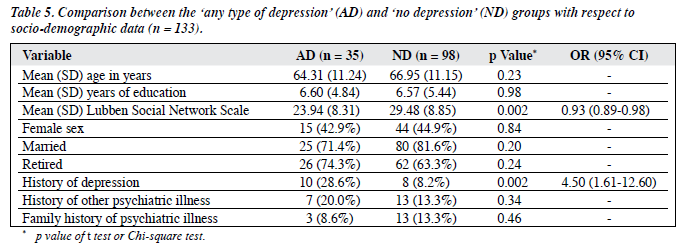
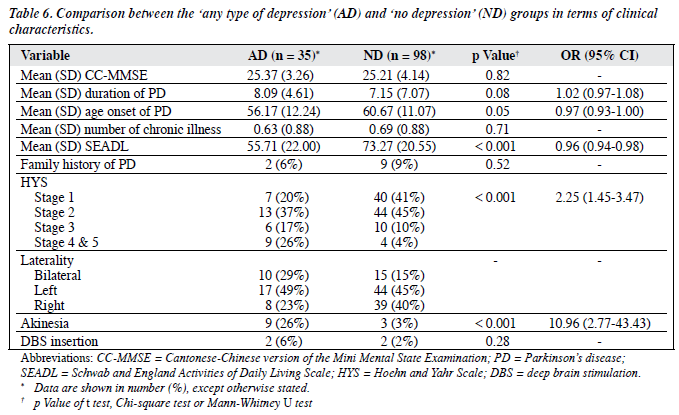
There was no statistical difference between the AD and ND groups in all the socio-demographic variables except for the history of depression. The AD group had a statistically significantly higher frequency of a history of depression (Chi-square test, p = 0.002), with an odds ratio (OR) of 4.5 (95% CI, 1.61-12.60). The AD group had significantly lower scores for the LSNS (Table 5).
Subjects in the AD group had a significantly younger age at onset of PD, more severe akinesia, and a higher HYS than did the ND subjects. The OR for the age of onset of PD was 0.97 (95% CI, 0.93-1.00). The OR for HYS was 2.25 (95% CI, 1.45-3.47). The OR for akinesia was high, at 10.96 (95% CI, 2.77-43.43). The AD group had a longer duration of PD, which was marginally significant. The AD group was more globally disabled as reflected by their lower SEADL scores (Table 6).
There were no statistical differences between the AD and ND groups with respect to the use of Madopar (Roche, Basel, Switzerland), Sinemet (Merck Sharp & Dohme, Glattbrugg, Switzerland), bromocriptine, and selegiline. There was a marginally significant difference between the 2 groups for the use of catechol-o-methyltransferase inhibitor (COMT inhibitor) medication, with an OR of 2.213 (95% CI, 0.89-5.54).
Correlations between Clinical Variables and Depression
The following variables were selected for analysis (p < 0.1): history of depression, duration of PD, age of onset of PD, HYS, akinesia, use of COMT inhibitor, LSNS, and SEADL. The above-mentioned variables were entered into the binary logistic regression with backward and forward stepwise methods and AD/ND as the dependent variables. A history of depression (p = 0.01), younger age of onset of PD (p = 0.03), and lower SEADL scores (p < 0.001) were found to be the clinical correlates of dPD. The results were consistent in both forward and backward mode.
Discussion
In a recent review, the average point prevalence for any type of dPD was found to be 31%.18 In our study, the point prevalence of all types of depressive disorder was 26.3%, which is generally in line with the review. The prevalence of dPD depends on the instruments that are used to detect depression. Studies that establish diagnoses of depressive disorders by using a SCID tend to report lower rates of depression.19
The frequency of dPD would equally be affected by the sample selection. The point prevalence of MDE in this study was higher than the corresponding figures in 2 community- based studies.19,20 As the rate of depression is likely to be related to the severity of PD, a sample from neurology clinics is likely to yield more severe PD than would a community- based one, and hence a higher frequency of depression. The results of this study agree better with those from a previous study that also recruited subjects from an outpatient clinic and used the same diagnostic instrument.21 The point prevalence of AD in that study, by Weintraub et al,21 was 29.7% and it was 26.3% in our study.
As there is a close association between cognitive impairment and dPD, excluding those patients with a CC-MMSE lower than 15 is not a perfect method. It is a convenient bedside method for detecting cognitive impairment but it cannot provide accurate staging in dementia. Thus, subjects with mild dementia co-morbid with PD might be missed, making this a limitation of this study. This probably partially explained the difference in point prevalence of MD between an earlier study and our study, as the former included all patients irrespective of their cognitive state, therefore yielding a higher point prevalence (25% vs 14.3%).22 Chinese patients, especially older ones, may present with somatic symptoms that may not be detected by the CB-SCID-I/P23 and this may partially explain the lower frequency of dPD in this study, compared with western counterparts.
Studies conducted in Taiwan and China have provided preliminary results24-26 on depression in Chinese PD patients. The point prevalence of any type of depression ranged from 38% to 56.4% in these studies. The point prevalence of dPD had a range of 38% to 56.4%. Many of the Chinese studies used low cut-off scores from the Hamilton Depression Rating Scale for diagnosing dPD leading to an inevitable overestimation of the frequency of dPD. The clinical correlates of dPD found by our study are: a history of depression, younger age of onset of PD, and lower SEADL score. The AD group had a significantly higher frequency of akinesia, more advanced HYS, and less social support than the ND group. Akinesia and HYS, which depicted the severity of PD, expressed their effects through the SEADL, which was found to be the clinical correlate of dPD. There is evidence to suggest that disability and dPD exert an effect on each other, especially in longitudinal studies.4,27
Therefore, the causal relationship between SEADL and dPD is complex.
In our study, cognitive impairment was not included as a clinical correlate of dPD, as the relationship between depression and cognitive impairment in PD is too complex to determine in a cross-sectional study. Depression may increase cognitive impairment in PD.28 It has been suggested that pre-existing depression often precedes the development of a dementia syndrome.29 There may be a common underlying disturbance in the frontal-subcortical system that leads to both depression and cognitive impairment.30
An improvement in depressive symptoms also improves MMSE.31 It has been suggested that the combination of depressive and anxiety symptoms may represent a specific depressive subtype in PD.32 In this study, the AD group suffered from a higher level of anxiety symptoms, as reflected by the higher scores in the Hospital Anxiety and Depression Scale–Anxiety Subscale.
In this study, subjects were identified by their attending neurologists, using the UKPDSBB diagnostic criteria, ensuring a sensitive diagnosis of PD and a homogenous sample. This was a multi-centre study involving a range of locations, making it likely that a more representative sample of PD patients attending neurology clinics in Hong Kong was collected. The CB-SCID-I/P, used to establish psychiatric diagnoses, has been shown by previous studies to be a reliable instrument for diagnosing mood disorders in a Hong Kong Chinese population.17 There are several limitations to this study. As only patients from neurology clinics were recruited, the spectrum of PD patients investigated is limited. Patients with PD being managed in general outpatient clinics and general medical outpatient clinics were not included. Hence, these results may not be generalisable to other settings. The sample size of this study is not adequate for a more detailed analysis in several respects. As the medications being taken by patients in the sample were diverse and the sample size for each medication was too small to test for statistical significance, the medications were grouped as antiparkinsonian medications and antihypertensive medications and their effects then measured. For the same reasons, only the number of chronic diseases was used in the analysis instead of individual physical co-morbidity. Therefore, the effect of physical co- morbidity could only be estimated. Furthermore, the sample size in this study was not adequate for a separate analysis of MDE, MinD, and dysthymia. Although clinical correlates have been identified, no temporal or possibly causal relationship between depression and clinical correlates could be demonstrated due to the cross-sectional nature of the study. A cohort should be investigated prospectively to determine the risk factors for depression in future studies. Our study could have been improved by recruiting controls with other neurological diseases along with the PD subjects.
This study found that the frequency of dPD in patients attending the 3 neurology clinics in Hong Kong is high. As treatment for dPD is readily available and is likely to improve the quality of life of PD patients, routine screening for dPD in neurology clinics is indicated. This study identified several clinical correlates of dPD that should enable physicians to identify high-risk patients. A liaison service assisting neurology clinics is a possible step that could be taken in the future to improve patient care. This study sends a clear message that more attention needs to be paid to dPD.
References
- Marsh L. Neuropsychiatric aspects of Parkinson’s disease. Psychosomatics 2000;41:15-23.
- Ho SC, Woo J, Lee CM. Epidemiologic study of Parkinson’s disease in Hong Kong. Neurology 1989;39:1314-8.
- Nilsson FM, Kessing LV, Sorensen TM, Andersen PK, Bolwig TG. Major depressive disorder in Parkinson’s disease: a register-based study. Acta Psychiatr Scand 2002;106:202-11.
- Brown RG, MacCarthy B, Gotham AM, Der GJ, Marsden CD. Depression and disability in Parkinson’s disease: a follow-up of 132 cases. Psychol Med 1988;18:49-55.
- Nilsson FM, Kessing LV, Bolwig TG. Increased risk of developing Parkinson’s disease for patients with major affective disorder: a register study. Acta Psychiatr Scand 2001;104:380-6.
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res 1983;275:321-8.
- Mayeux R, Stern Y, Sano M, Williams JB, Cote LJ. The relationship of serotonin to depression in Parkinson’s disease. Mov Disord 1988;3:237-44.
- Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, et al. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1985;48:413-21.
- Perry R, McKeith I, Perry E. Lewy body dementia—clinical, pathological and neurochemical interconnections. J Neural Transm Suppl 1997;51:95-109.
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745-52.
- Chiu HF, Lee HC, Chung WS, Kwong PK. Reliability and validity of the Cantonese version of Mini-Mental State Examination — a preliminary study. Hong Kong J Psychiatry 1994;4:25-8.
- Lam S. The health of the elderly in Hong Kong. Hong Kong: Hong Kong University Press; 1997.
- Tandeter HB, Shvartzman P. Parkinson’s disease camouflaging early signs of hypothyroidism. Postgrad Med 1993;94:187-90.
- Lubben JE. Assessing social networks among elderly populations. Fam Community Health 1988;11:42-52.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42.
- Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillinghan FH, Donaldson MC, editors. Third Symposium on Parkinson’s Disease. Edinburgh: Livingston; 1969:152-7.
- So E, Kam I, Liu Z, Fong S, Chung D, Leung CM. The Chinese bilingual SCID-I/P project: stage I — reliability for mood disorders and schizophrenia. Hong Kong J Psychiatry 2003;13:7-18.
- Slaughter JR, Slaughter KA, Nichols D, Holmes SE, Martens MP. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001;13:187-96.
- Tandberg E, Larsen JP, Aarsland D, Cummings JL. The occurrence of depression in Parkinson’s disease: A community-based study. Arch Neurol 1996;53:175-9.
- Hantz P, Caradoc-Davies G, Caradoc-Davies T, Weatherall M, Dixon G. Depression in Parkinson’s disease. Am J Psychiatry 1994;151:1010- 4.
- Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc 2004;52:784-8.
- Leentjens AF, Lousberg R, Verhey FR. Markers for depression in Parkinson’s disease. Acta Psychiatr Scand 2002;106:196-201.
- Lee S, Yu H, Wing Y, Chan C, Lee AM, Lee DT, et al. Psychiatric morbidity and illness experience of primary care patients with chronic fatigue in Hong Kong. Am J Psychiatry 2000;157:380-4.
- Chen ZM, Zhang LS, Guo BY. The co-morbidity of depression in Parkinson’s disease. Chinese Journal of Nervous and Mental Disease 2003;29:140-1.
- Dong Q, Li YS. Studies on the distribution of Psychiatric Disturbances in Patients with Parkinson’s disease. Nervous Diseases and Mental Health 2004;4:440-1.
- Wu FP, Niu XQ, Shi HP. Clinical factors related to depression in Parkinson’s disease. Factors related to depression in Parkinson’s disease. Chinese Journal of Clinical Rehabilitation 2004;5:1901.
- Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 1992;55:377-82.
- Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment, and Parkinson disease. Neurology 1981;31:645-50.
- Hughes TA, Ross HF, Mindham RH, Spokes EG. Mortality in Parkinson’s disease and its association with dementia and depression. Acta Neurol Scand 2004;110:118-23.
- Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry 1992;149:443-54.
- Rojo A, Aguilar M, Garolera MT, Cubo E, Navas I, Quintana S. Depression in Parkinson’s disease: clinical correlates and outcome. Parkinsonism Relat Disord 2003;10:23-8.
- Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J 2001;77:89-93.