East Asian Arch Psychiatry 2012;22:7-11
ORIGINAL ARTICLE
Serum Prolactin Levels and the Acute-phase Efficacy in Drug-naïve Schizophrenia Treated with Ziprasidone and Olanzapine (translated version)
Dr Xiaoli Wu, Department of Psychology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, PR China.
Dr Ji-Hui Wang, MApplPsych, Department of Psychology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, PR China.
Dr San-Hong Hu, MApplPsych, Department of Psychology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, PR China.
Dr Jiong Tao, Department of Psychology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, PR China.
Address for correspondence: Dr Jiong Tao, Department of Psychology, The Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, PR China.
Tel: (86) 13600071635; Fax: (86-20) 8756 3011; email: tj2023@163.com
Submitted: 25 August 2011; Accepted: 4 November 2011
Abstract
Objectives: To study the efficacy and associated serum prolactin levels of ziprasidone and olanzapine treatment in drug-naïve schizophrenia patients.
Methods: All 78 inpatients with drug-naïve schizophrenia were recruited from the Department of Psychology, The Third Affiliated Hospital of Sun Yat-sen University. They were divided into either olanzapine group (n = 49 [24 men, 25 women]; mean [standard deviation] age, 24 [6] years) or ziprasidone group (n = 29 [14 men, 15 women]; mean [standard deviation] age, 23 [7] years), all of whom were treated for 4 weeks. The serum prolactin level, the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impression–Severity (CGI-S), and Clinical Global Impression–Improvement scores were measured before and at the end of treatment.
Results: In the olanzapine group, the respective mean (standard deviation) PANSS and CGI-S scores after the treatment (62 ± 15 and 3 ± 1) were significantly lower than those before the treatment (104 ± 14 and 6± 1) [p < 0.01]. In the ziprasidone group, the corresponding scores after the treatment (75 ± 20 and 4 ± 1) were also significantly lower than those before the treatment (104 ± 17 and 6 ± 1) [p < 0.01]. The decreases in mean (standard deviation) PANSS total (42 ± 17) and PANSS positive scores (12 ± 6) in the olanzapine group were significantly higher than those in the ziprasidone group (29 ± 12 and 6 ± 4, respectively) [p < 0.01]. The increase of serum prolactin in the ziprasidone female group (47 ± 51 μg/L) was significantly higher than that in the ziprasidone male group (17 ± 11 μg/L), the olanzapine male group (5 ± 16 μg/L), and the olanzapine female group (21 ± 34 μg/L) [p < 0.05].
Conclusions: Both ziprasidone and olanzapine are effective for treating drug-naïve acute schizophrenia, but olanzapine was superior to ziprasidone in terms of positive and general psychopathological symptoms. In women, ziprasidone was associated with greater changes in prolactin level than olanzapine.
Keywords: Antipsychotic agents; Prolactin; Schizophrenia
Introduction
Ziprasidone is a new atypical antipsychotic drug approved for use in the United States in 2001 and in Mainland China in 2007. Olanzapine was approved for use in the United States in 1996 and in Mainland China in 1999. Worldwide, both drugs are currently first-line drugs for the treatment of schizophrenia, as they are efficacious in terms of treating the positive, negative and general psychopathological symptoms.1-4 Ziprasidone has minimal effect on body mass and glycolipid metabolism which is not the case for olanzapine, but the effects of the 2 drugs on prolactin levels were smaller than those of traditional antipsychotics and the atypical antipsychotic risperidone. To date, domestic clinical studies directly comparing the effects of ziprasidone and olanzapine on prolactin levels have been rare, but case reports of clinical associations were not uncommon. This study was therefore initiated to compare the efficacy of ziprasidone and olanzapine on drug-naïve acute schizophrenia and their effects on serum prolactin levels.
Subjects and Methods
Study Subjects
The subjects of this study were inpatients at the Department of Psychology of the Third Affiliated Hospital, Sun Yat-sen University during the period March 2010 to February 2011. The study was approved by the local ethics committee, and was conducted in accordance with the World Medical Association’s Declaration of Helsinki. The inclusion criteria were: (1) fulfilment of the diagnostic criteria for schizophrenia stated in the third version of the Chinese Classification and Diagnostic Criteria of Mental Disorders; (2) being antipsychotic drug-naïve; (3) giving written informed consent; (4) aged being 18 to 35 years; and (5) having a Positive and Negative Syndrome Scale (PANSS) score of ≥ 60. Exclusion criteria were: (1) presence of any severe physical illness (particularly gynaecological and endocrine diseases); (2) a history of drug and alcohol dependence; and (3) being pregnant or breast-feeding within the last 2 years. Among the 57 patients receiving olanzapine treatment, 8 dropped out for various reasons, so only the remaining 49 patients were included in the analysis. Among the 36 patients receiving ziprasidone treatment, 7 dropped out for various reasons, leaving the remaining 29 patients in the final analysis. Thus, a total of 78 patients were included in this study.
The olanzapine group comprised 49 patients, in whom 24 were men and 25 were women. Their ages ranged from 18 to 35 years, with a mean (± standard deviation [SD]) of 24 ± 6 years and a mean (± SD) disease duration of 1 ± 1 years. The ziprasidone group included 29 patients in whom 14 were men and 15 were women. Their ages ranged from 18 to 35 years, with a mean (± SD) of 23 ± 7 years and a mean (± SD) disease duration of 3 ± 3 years. Differences in gender composition, age, baseline PANSS total score, baseline Clinical Global Impression–Severity (CGI-S) score, and baseline prolactin levels between the olanzapine and ziprasidone groups bore no statistical significance (p > 0.05); length of disease of the ziprasidone group was longer than that of the olanzapine group, with the difference being statistically significant (t = 2.90, p = 0.01), but there was no correlation between the observed indicators during the course of the disease and after the treatment (p > 0.05).
The study subjects were further divided by gender into the olanzapine male group, olanzapine female group, ziprasidone male group, and ziprasidone female group. Differences in the length of hospital stay, baseline PANSS total, and baseline CGI-S scores among the 4 groups were not statistically significant (p > 0.05); differences in age (F = 2.89, p = 0.04), length of disease (F = 2.99, p = 0.04), and baseline prolactin levels (F = 10.97, p = 0.00) of the 4 groups were statistically significant. There was no correlation between age, length of disease, baseline prolactin levels, and increased prolactin levels after the treatment (p > 0.05).
Study Design
This was a randomised controlled, open-label clinical observational trial, with a period of outcome observation lasting 4 weeks.
The olanzapine group received olanzapine (Zyprexa; Lilly USA, LLC., 5 mg / tablet): initial dose of 5-10 mg/d, increased to 15-30 mg/d within 1 to 2 weeks, with the mean (± SD) dose of 23 ± 4 mg/d. The ziprasidone group received ziprasidone (Zeldox; Pfizer Inc., USA, 40 mg / tablet): initial dose of 40 to 80 mg/d, and increased to 160 to 200 mg/d within 1 to 2 weeks, with the mean (± SD) dose being 168± 17 mg/d. Trihexyphenidyl hydrochloride was used in combination in subjects with extrapyramidal symptoms, whereas clonazepam was used in combination in subjects with severe insomnia. Efficacy observation and assessment of prolactin levels, at baseline and after the treatment, were conducted through PANSS, CGI-S, and Clinical Global Impression–Improvement (CGI-I) measurements. Serum prolactin level was measured once at baseline and once after the treatment, for which 8 mL of venous blood was collected at 8 am in the morning after fasting. Plasma from each sample was separated and stored at -20°C; later the serum prolactin level was detected by radioimmunoassay.
Statistical analysis was carried out using SPSS 16.0 software, and a p value of ≤ 0.05 was regarded as denoting a statistically significant difference. Continuous data were described using means and SDs and analysed using t tests. Rate comparisons were examined using the χ2 tests. Comparison of means between multiple groups entailed analysis of variance.
Results
For various reasons, there were 8 (14%) dropouts from the olanzapine group and 7 (19%) in the ziprasidone group. In the olanzapine group, 5 (10%) used clonazepam in combination, while 22 (45%) used trihexyphenidyl in combination. In the ziprasidone group, 4 (14%) used clonazepam in combination, while 20 (65%) used trihexyphenidyl in combination. Differences in the rate of combined medication use in the 2 groups were not statistically significant (p > 0.05).
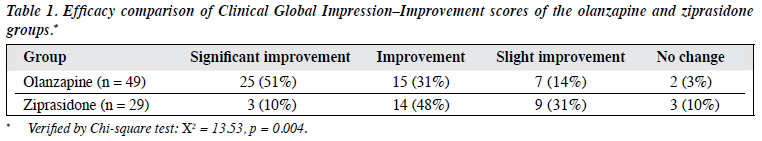
The post-treatment CGI-I scores of the olanzapine group were superior to those in the ziprasidone group, the difference being statistically significant (p < 0.01) [Table 1].
Comparison of Positive and Negative Syndrome and Clinical Global Impression–Severity Scores
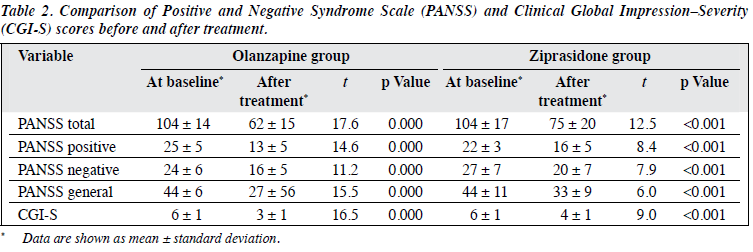
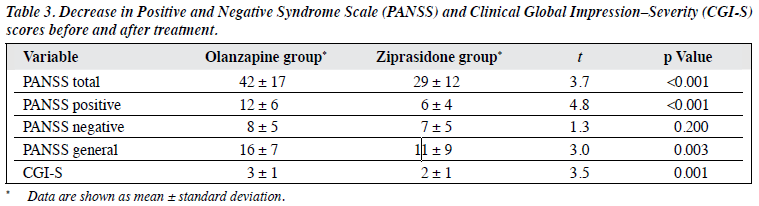
The various scale scores of the olanzapine and ziprasidone groups after the treatment were significantly lower than those at baseline, all differences being statistically significant (p < 0.01). The decreases in post-treatment PANSS total, PANSS positive, PANSS general psychopathological subscale, as well as CGI-S scores in the olanzapine group were greater than that in the ziprasidone group, all differences being statistically significant (p < 0.05). Differences in the decreases in PANSS subscale scores in the 2 groups were not statistically significant (p > 0.05; Tables 2 and 3).
Gender Differences in Change in Prolactin Levels
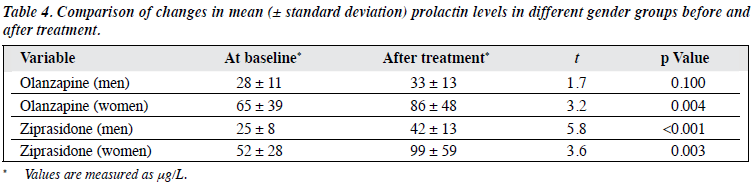
Post-treatment prolactin levels of women in the olanzapine group were higher than that at baseline, as were post- treatment prolactin levels of men and women in the ziprasidone group, all differences being statistically significant (p < 0.01). Analysis of variance conducted on the increases in prolactin levels in the 4 groups yielded significant results. Based on means and SDs, differences between the 4 groups (olanzapine male [5 ± 16 μg/L], olanzapine female [21 ± 34 μg/L], ziprasidone male [17± 11 μg/L], ziprasidone female [47 ± 51 μg/L]) were statistically significant (F = 5.47, p = 0.002). Further comparison between the groups illustrated that only the increase of prolactin levels in ziprasidone-treated women was greater than that in the other 3 groups, the difference being statistically significant (p < 0.05; Table 4).
Discussion
In this study both ziprasidone and olanzapine provided effective treatment for acute schizophrenia. The slightly greater efficacy of olanzapine was primarily reflected in the significantly greater decreases of PANSS total scores and subscale scores, CGI-S scores after the treatment (p < 0.05) and CGI score improvements. This finding was consistent with that reported by Komossa et al,5 who conducted a meta-analysis of randomised controlled trials on ziprasidone and other atypical antipsychotics for schizophrenia based on 9 articles, 4 of which entailed comparisons of ziprasidone and olanzapine and included 1291 patients. However, with their overall dropout rate as high as 59%, the persuasiveness of Komossa et al’s analysis5 was limited. In contrast, the dropout rate from this study was less than 20% and none of the participants had received prior antipsychotic drug treatment. The slightly greater overall efficacy of olanzapine treatment could be related to its better and faster impact on positive and general psychopathological symptoms. Thus, further analysis demonstrated that the decreases in total PANSS scores, PANSS positive subscale scores, and PANSS general psychopathological scores in the olanzapine group after 4 weeks of treatment were greater than that in subjects of ziprasidone group. Research conducted locally by Chen et al6 also confirmed the faster onset of olanzapine’s effect on positive symptoms, suggesting that it had certain advantages for the improvement of positive and general psychopathological symptoms in drug-naïve patients with acute schizophrenia. This could also partly explain the lower dropout rate in the olanzapine than ziprasidone group of this study. Since complaints of patients with acute schizophrenia were often associated with positive symptoms and physical discomfort, improvement in positive and general psychopathological symptoms might be one of the important reasons why patients are motivated to adhere to the treatment in the acute phase of their illness.
Serum prolactin is a hormone secreted by the anterior pituitary gland. Its secretion is regulated via a dopamine (DA) negative feedback loop in the funnel nodules of the hypothalamus. Atypical antipsychotic drugs function by blocking the 5HT2 and DA receptors in the mesolimbic and mesocortical pathways; they block DA receptors in the funnel nodules of the hypothalamus to varying extents and result in elevated prolactin levels.7
According to the literature,8 the order of activity of atypical antipsychotic drugs on serum prolactin levels can be summarised as: risperidone > olanzapine > quetiapine > aripiprazole. The current study showed that olanzapine and ziprasidone were both capable of increasing serum prolactin levels significantly (p < 0.01), and counters the claim that “ziprasidone had minimal impact on serum prolactin levels” reported by Stroup et al9 and Zhang et al.4 Possible reasons for the discrepancy were as follows: (1) Our study subjects were different, being antipsychotic drug–naïve schizophrenic patients, while in other studies they were not. Therefore, baseline prolactin levels could have been significantly increased by previous medication. (2) Ours was an independent research project not funded by any pharmaceutical company, and therefore liable to fewer biases. (3) The majority of previous studies tended to compare the 2 medications with drugs known to have a significant influence on serum prolactin levels (e.g. first- generation antipsychotics and the atypical antipsychotic risperidone). A few studies directly compared the effects of olanzapine, ziprasidone and quetiapine on serum prolactin levels, giving the impression that ziprasidone and olanzapine only had minimal effects on serum prolactin levels.
According to the studies of Grootens et al,10 the effects of ziprasidone and olanzapine on serum prolactin levels in schizophrenic patients yielded no statistically significant difference. Nevertheless, our study underlined a slight difference; only women in the olanzapine group demonstrated elevated serum prolactin levels, and ziprasidone groups of both genders showed elevated serum prolactin levels, with differences before and after the treatment being statistically significant. Such difference in results could be due to patient selection. Patients selected for Grootens et al’s study10 received antipsychotic drug treatment before entering their study and no stratification by gender. Results of the research by Zhang et al4 also clearly illustrated that despite the slightly reduced mean prolactin levels of the ziprasidone group at the end of 6 weeks of treatment, the prolactin levels in 43% of the males and 60% of the females with normal baseline levels showed abnormal increases at the end of the treatment. The prolactin levels of 60% of the subjects with abnormal baseline prolactin levels remained abnormal at the end of the treatment. These results imply that the mechanism influencing the prolactin secretion is too complex to be fully explained with a single theory involving DA receptors. Due to the unique functional characteristics of these receptors, ziprasidone and olanzapine as atypical antipsychotics,11,12 their effects on the DA receptors in the funnel nodules of the hypothalamus are in theory minor, and should be less likely to elevate serum prolactin levels. However, this proposition cannot be substantiated by the results of our study. Our study also showed that the effects of ziprasidone and olanzapine on the serum prolactin levels in women were greater than those in men. This observation is consistent with the findings in clinical practice, and implies the greater vulnerability of the female endocrine system to exogenous drug interference.
Shortcomings of this study included: our relatively small sample size, a relatively short duration of observation, a narrow age span in the study subjects, absence of positive control drugs, and absence of a double-blind experimental design. Confirmation of these findings should be pursued through an independent, large-sample, long-term study with a better design.
In summary, both ziprasidone and olanzapine are effective in treating drug-naïve patients with acute schizophrenia. In women, ziprasidone was associated with greater changes in prolactin level than olanzapine. The effects of the 2 drugs on serum prolactin levels in women were more significant than those in men. These findings imply that more attention needs to be accorded to the endocrine effects of atypical antipsychotic drugs, so as to better individualised treatment using a “holistic treatment approach”.
Declaration
The authors declare no conflict of interest.
Acknowledgements
Funding for this study was provided by the Science and Technology Plan Project (2009B080701080) of Guangdong Province. The authors would like to gratefully acknowledge the contributions of all of the doctors, nurses, technicians, and patients that participated in this study. The study was not sponsored by any pharmaceutical company.
References
- Simpson GM, Glick ID, Weiden PI, Romano SJ, Siu CO. Randomized, controlled, double-blind multicenter comparison of the efficacy and tolerability of ziprasidone and olanzapine in acutely ill inpatients with schizophrenia or schizoaffective disorder. Am J Psychiatr 2004;161:1837-47.
- Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, et al. Change in metabolic syndrome parameters with antipsychotics treatment in the CATIE Schizophrenia Trial: prospective data from phase I. Schizophr Res 2008;101:273-86.
- Addington DE, Labelle A, Kulkarni J, Johnson G, Loebel A, Mandel FS. A comparison of ziprasidone and risperidone in the long-term treatment of schizophrenia: a 44-week, double-blind, continuation study. Can J Psychiatry 2009;54:46-54.
- Zhang HY, Liu C, Yan J, Shu L, Li HF, Gu NF, et al. The efficacy and safety of ziprasidone in the treatment of acute exacerbation of schizophrenia [in Chinese]. Chinese Journal of New Drugs 2010;19:2076-80.
- Komossa K, Rummel-Kluge C, Hunger H, Schwarz S, Bhoopathi PS, Kissling W, et al. Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2009;(4):CD006627.
- Chen Q, Liu XL, Chen YZ. Clinical observation of ziprasidone and olanzapine treatment of schizophrenia [in Chinese]. Chinese Journal of Hospital Pharmacy 2008;28:1856-8.
- Halbreich U, Kinon BJ, Gilmore JA, Kahn LS. Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology 2003;28:Suppl 1:S53-67.
- Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol 2008;23:201-9.
- Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22.
- Grootens KP, van Veelen NM, Peuskens J, Sabbe BG, Thys E, Buitelaar JK, et al. Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial. Schizophr Bull 2011;37:352-61.
- Schmidt AW, Lebel LA, Howard HR Jr, Zorn SH. Ziprasidone: a novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol 2001;425:197-201.
- Bhana N, Foster RH, Olney R, Plosker GL. Olanzapine: an updated review of its use in the management of schizophrenia. Drugs 2001;61:111-61.