East Asian Arch Psychiatry 2015;25:64-72
ORIGINAL ARTICLE
Dr Richa Tripathi, MD, Department of Psychiatry, Sawai Man Singh Medical College, Jaipur, India.
Dr Ajitabh Soni, MD, Department of Psychiatry, Sawai Man Singh Medical College, Jaipur, India.
Prof. Alok Tyagi, MD, Unit Head, Department of Psychiatry, Sawai Man Singh Medical College, Jaipur, India.
Dr Shubham Mehta, MD, Consultant Psychiatrist, Navjeevan Hospital, Hisar, Haryana, India.
Prof. Suresh Gupta, MD, Department of Psychiatry, Sawai Man Singh Medical College, Jaipur, India.
Address for correspondence: Dr Richa Tripathi, NMHP Trainees Hostel, Room 4, Psychiatric Center Jaipur, Jaipur 302004, India.
Tel: (91) 8290996876; Email: drricha12@gmail.com
Submitted: 3 November 2014; Accepted: 24 December 2014
Abstract
Objective: The primary objective of this study was to examine neurological soft signs in patients with obsessive-compulsive disorder compared with patients with schizophrenia and a control group in the Indian setting. The secondary objective was to find any correlation between age at onset and neurological soft signs scores, as well as that between severity of obsessive-compulsive disorder symptoms (total Yale-Brown Obsessive Compulsive Scale score) and neurological soft signs scores.
Methods: This was a cross-sectional hospital-based study of 135 individuals (45 patients with schizophrenia, 45 patients with obsessive-compulsive disorder who were attending the psychiatric outpatient department of Sawai Man Singh Medical College, Jaipur, India, and 45 matched healthy controls) from 20 June 2013 to 22 December 2014. After applying strict inclusion and exclusion criteria, the participants completed the study instruments (Cambridge Neurological Inventory [Part 2] and Yale- Brown Obsessive Compulsive Scale). Their socio-demographic data were also recorded.
Results: The neurological soft signs total score and domain scores (motor coordination, sensory integration, and disinhibition) were significantly higher in patients with schizophrenia (p < 0.05) than in the obsessive-compulsive disorder group or the control group. The obsessive-compulsive disorder group did not significantly differ from the control group in terms of neurological soft signs scores. No correlation was found between neurological soft signs scores and age at onset as well as that between neurological soft signs scores and total Yale-Brown Obsessive Compulsive Scale score.
Conclusion: Neurological soft signs assessed by the Cambridge Neurological Inventory and Yale-Brown Obsessive Compulsive Scale, which discriminate patients with schizophrenia from controls, appear to be relatively specific to schizophrenia. Further studies are required to explore neurological soft signs in patients with obsessive-compulsive disorder.
Key words: Neurologic examination; Psychiatric Status Rating Scales; Schizophrenia
Introduction
Obsessive-compulsive disorder (OCD) is characterised by obsessions, including recurrent, intrusive, and unwanted thoughts, images, or impulses, and compulsions, consisting of repetitive behaviours or thoughts that can lead to significant disability.1,2 Obsessive-compulsive disorder has traditionally been regarded as a functional neurosis and has been included as ‘Obsessive Compulsive And Related Disorders’ in the DSM-V.
Recently, however, an alternative view in which OCD is seen as primarily a neurological motor disorder has become prominent.3-7 There is evidence linking neurological dysfunction to OCD, such as onset of OCD following head trauma, encephalitis, or streptococcal infections.8 Abnormalities in the orbital frontal cortex, anterior cingulate, caudate, and thalamus have been demonstrated in OCD patients, suggesting dysfunction of fronto-subcortical circuitry.9 Further important evidence of abnormal neurological involvement in OCD is the above- normal frequency of neurological soft signs (NSS) in this population.10-14 Neurological soft signs, in contrast to hard neurological signs, are subtle neurological non-localising anomalies, including altered motor coordination, balance, motor integration, and sensory integration. Neurological soft signs may reflect cerebral dysfunction in distributed neural networks, and they can give additional information on the functional organisation that characterises some psychiatric disorders.15 Neurological soft signs have been described in several psychiatric disorders, but they are particularly prevalent in schizophrenia.16 Only a few studies have explored NSS in patients with OCD, and their results are contradictory. Literature reviews on NSS in OCD showed inconsistency in the results. Some found a higher prevalence of NSS in OCD patients than in healthy controls11,12,17-24 including impaired motor coordination11,12,20-22 sensory integration abnormalities,17,22,23 and presence of primitive reflexes.25 However, other studies did not find any differences between OCD patients and controls in either NSS total scores25,26 or in specific NSS domains such as motor coordination,17,24,26 sensory integration,11,26 involuntary movements,26 or primitive reflexes.23 Like many psychiatric disorders, OCD is heterogeneous, and previous studies have stressed that patients with early-onset OCD are more likely to be male, experience more severe symptoms than females in their age-group, and have a family member with OCD than those with later-onset OCD.27 This suggests that early age at onset of OCD reflects more neurodevelopmental loading than does later age at onset. Prevalence of NSS may be higher in patients with early age at onset of OCD than in patients with later age at onset.
With this in mind, our primary objective in this study was to examine NSS in patients with OCD and compared them with patients with schizophrenia and a healthy control group in the Indian setting. A secondary objective was to find any correlation between age at onset of OCD patients and NSS scores, as well as severity of OCD symptoms and NSS scores.
Methods
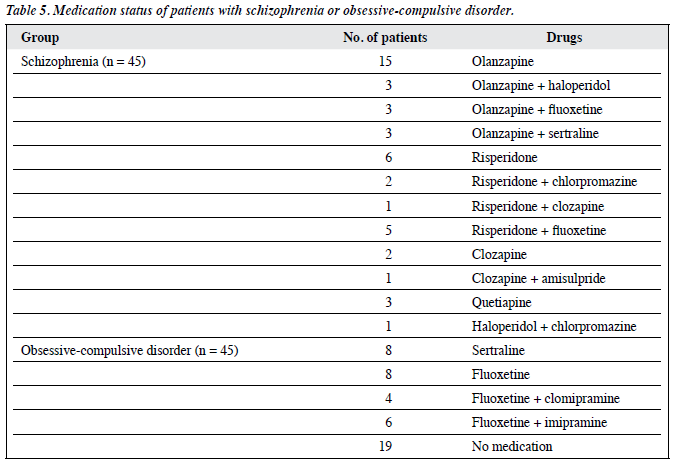
A cross-sectional hospital-based study was conducted at the Department of Psychiatry (Psychiatric Center Jaipur) of the tertiary care hospital Sawai Man Singh Hospital and affiliated Medical College, Jaipur, India. Ethical approval for the study was obtained from the Institutional Ethics Committee. For an alpha error of 0.05% and power of study of 80%, we enrolled 90 consecutive patients (45 with schizophrenia and 45 with OCD) who consented to the study and 45 healthy controls over a period of 1.5 years from 20 June 2013 to 22 December 2014. The diagnosis of OCD was made using the Composite International Diagnostic Interview-328 (patients aged 18-55 years) for patients who met the criteria for OCD, but not those for schizophrenia. Patients with schizophrenia (aged 18-55 years) met the ICD-10 criteria for schizophrenia, but not those for OCD. All the schizophrenic patients were taking medication and 26 patients in the OCD group were taking medication. Patients with mental retardation, significant neurological or medical disease, and co-morbid psychiatric disorder were excluded from the study.
After taking the written informed consent for participation in the study, the patients underwent evaluation by the study tools. The control participants were volunteers recruited from among the hospital personnel and attendants of patients who were not their first-degree relative and who had similar socio-demographic characteristics (i.e. age, sex, education) to the patient sample.
Assessment Tools
All participants’ socio-demographic characteristics of age, sex, marital status, occupation, education status, religion, family type, and locality were recorded.
Yale-Brown Obsessive Compulsive Scale
The Yale-Brown Obsessive Compulsive Scale (YBOCS)29 is a 10-item scale, with each item rated from 0 (no symptoms) to 4 (extreme symptoms), yielding a total possible score range from 0 to 40. A total score of 0 to 7 was classified as subclinical; 8 to 15 as mild; 16 to 23 as moderate; 24 to 31 as severe; and 32 to 40 as extreme.
Cambridge Neurological Inventory
The Cambridge Neurological Inventory (Part 2) [CNI] is a standardised clinical examination of NSS described by Chen et al.30 The CNI was comprised of 3 subscales, including: (a) motor coordination (MC) involving finger- nose test, finger-thumb tapping, finger-thumb opposition, dysdiadochokinesia, fist-edge-palm test, and Oseretsky test; (b) sensory integration (SI) involving extinction, finger agnosia, stereognosis, agraphesthesia, and left-right orientation; and (c) disinhibition (DI) involving Go/No-go test. Each test was performed and rated as 0, 1, 2, where ‘0’ referred to normal; ‘1’ as 1 or 2 minor mistakes; and ‘2’ as major disruption. We did not test the reliability of the ratings, as only one person supervised the NSS tests among participants and this person was blinded to the diagnosis.
Statistical Analysis
Data were analysed using the Statistical Package for the Social Sciences Windows version 19.0 (IBM Corp, Armonk [NY], US). Descriptive statistics were expressed as mean ± standard deviation and frequency (percentage) as appropriate. We compared NSS scores (MC, SI, DI, and NSS total scores) between these 3 groups. Assuming normal distribution of values, analysis of variance and Chi-square test was used to compare these 3 groups and to ascertain significance of difference. Post-hoc test was later applied to judge which 2 groups had significant differences in direct comparison. Correlation coefficient was used to measure the relationship between YBOCS total score and NSS total score and subscores, and also between age at onset of OCD symptoms and NSS scores. The level of significance was set at p < 0.05.
Results
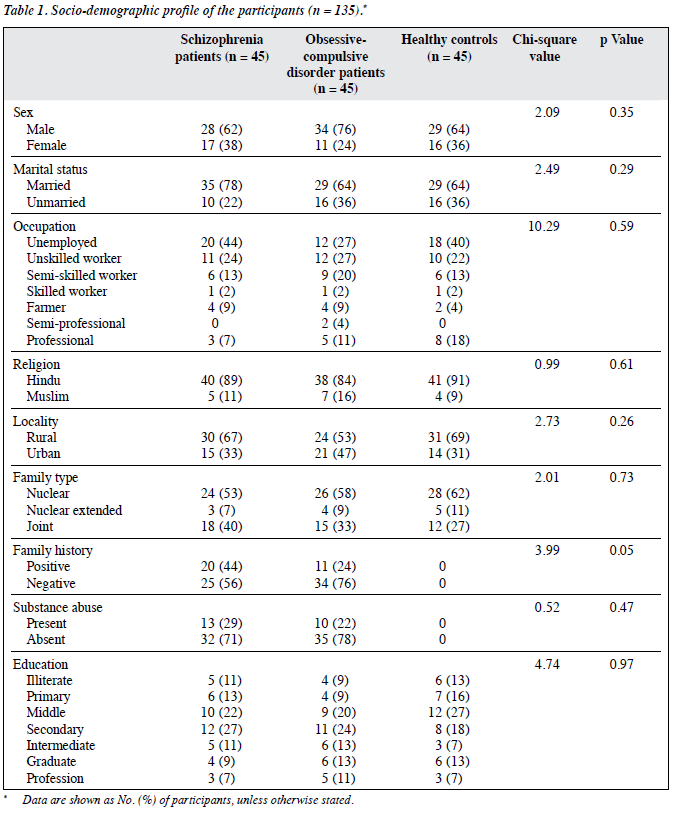
The socio-demographic and clinical characteristics of the groups are presented in Table 1. All 3 groups were comparable in terms of socio-demographic profile, with no significant differences. More of the patients were Hindu, married, and men of lower socio-economic status, residing in rural areas with a nuclear family. A total of 44% of schizophrenia patients and 27% of OCD patients were unemployed. Family history of psychiatric illness was present in 44% of patients with schizophrenia and 24% of those with OCD.
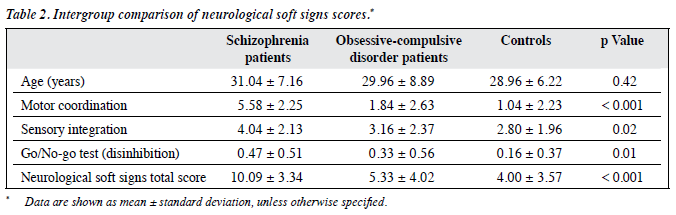
The mean age of the 3 groups was comparable (31.04 years in schizophrenia group, 29.96 years in the OCD group, and 28.96 years in the controls; Table 2). The differences were not statistically significant.
The NSS total score was significantly higher in patients with schizophrenia (10.09) than in OCD group (5.33) and the controls (4.00). The schizophrenic patients also had significantly higher MC, SI, and DI subscores compared with the other 2 groups (Table 2).
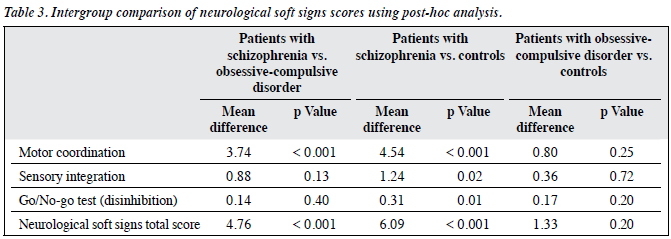
We further compared the 3 groups by using post-hoc analysis as shown in Table 3 and found that the schizophrenia group differed significantly in terms of NSS total (p < 0.001) and MC scores (p < 0.001) from the OCD group. There were significant differences between the schizophrenia group and the controls in NSS total (p < 0.001), MC (p < 0.001), SI (p = 0.02), and DI scores (p = 0.01). No significant differences were found between the OCD and the controls in NSS total scores or subscores (MC, SI, and DI).
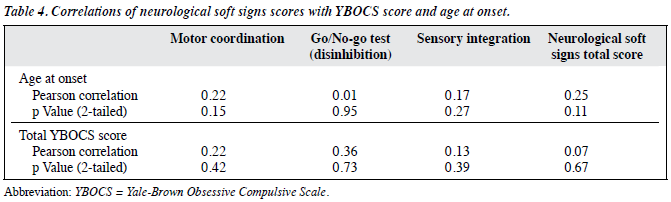
Table 4 shows that there was no significant correlation between total YBOCS score and NSS total score or subscores, as well as that between age at onset and NSS total scores or subscores.
Discussion
Both schizophrenia and OCD are thought to reflect abnormality in brain development, and NSS are considered to be a marker of abnormal neurodevelopment in patients with schizophrenia. Our main objective of this study was to find out whether NSS could also be a valid marker of deviant neurodevelopment in OCD patients.
We compared OCD patients with carefully matched patients with schizophrenia and healthy controls, and found higher NSS scores in patients with schizophrenia than in those with OCD and the controls. This was in accordance with the study by Jaafari et al25 on 162 participants (54 for each with schizophrenia, OCD, and healthy controls), and they found higher NSS scores in patients with schizophrenia than in those with OCD, who did not differ from the controls.
Stein et al26 and Bolton et al23 also reported similar findings. In the latter study involving 51 OCD patients, 47 schizophrenic patients and 67 healthy controls, results showed that in comparison with the OCD group, the schizophrenia group had raised levels of neurological signs, in particular CNI categories of hard signs, MC, tardive dyskinesia, catatonic signs, and extrapyramidal signs.23
However, they found similar levels for certain categories of NSS such as SI, primitive reflexes, and DI between schizophrenia and OCD group. In our study, significant difference was also found in MC, but not in SI and DI.
In a review of neurological signs in schizophrenia that did not include studies of OCD, Heinrichs and Buchanan18 concluded that schizophrenic patients have more neurological abnormalities than patients with mixed psychiatric disorders and affective disorders. Our results also suggest that NSS are more severe in patients with schizophrenia than in those with OCD.
We found no differences in NSS when comparing patients with OCD to healthy controls. Our results are in line with those by Guz and Aygun24 who investigated the relationship of NSS between patients with OCD and the control group using the Physical And Neurological Examination for Soft Signs (PANESS) scale31 and found that there were no significant differences between the patient and control groups in NSS except for graphesthesia, 2-point discrimination, and total PANESS scores. The differences in findings may be due to the different instruments used for NSS evaluation. Also, we did not do item-by-item analysis and had evaluated SI as a single entity.
Stein et al26 also reported similar findings when they compared female trichotillomania patients, OCD patients, and healthy controls, and found that the groups did not differ in NSS total scores. However, these researchers only studied women so the finding may be due to gender effects on NSS. The literature on sex and NSS in schizophrenia mostly report no difference in the prevalence of NSS between men and women.32-34 However, in a study including both schizophrenic patients and other psychiatric patients, men had more NSS than women.35
Contrary to our finding, Bolton et al23 found that OCD patients had higher NSS scores than the control group for MC, SI, and primitive reflexes. In this study,23 the instrument used to evaluate NSS (CNI) and the OCD group (54 participants) were similar to ours in terms of medication status (56% were taking medication), but there were no details on type of medication. In our study, 58% (26 out of 45) of OCD patients were receiving medication (Table 5) but we did not find significantly higher rates of NSS in OCD patients than in the controls. Our different finding to that of Bolton et al23 may be due to differences in subgroups of the study population, as Bolton et al23 had enrolled 24 patients from community advertisement while, in our study, all the patients were enrolled from a clinical setting. Similarly, other studies also found higher NSS scores in OCD patients than in control group.11,12,17-24 Our study did not replicate those findings. The contradictory findings could be partly explained by differences in medication status, different subgroups of population, differences in methodology, or different instrument use.
In our study we did not examine the influence of medication on NSS. This is a major limitation. The reason was because it was difficult to introduce, in a linear regression analysis, either chlorpromazine equivalents, as most of the OCD patients were not taking antipsychotic medication, or selective serotonin reuptake inhibitors dose equivalents, because most patients with schizophrenia did not take antidepressant medication, and the control participants were not taking any medication.
The literature regarding influence of medication on NSS varies. Some authors agree, while others refute the influence of medication on NSS. Lawrie et al36 suggested that SI abnormalities are non-specific markers of neurodevelopmental abnormality, which cannot be attributed to antipsychotic medication. Kolakowska et al37 concluded that the higher prevalence of NSS found among patients with schizophrenia, compared with healthy controls, is not simply a consequence of medication. Some studies found that NSS are present in neuroleptic-naïve patients presenting with first-episode schizophrenia,32,38 thus are unlikely to be sequelae of neuroleptic therapy.32,38,39 Contrary to this, some investigators have found significant correlations between NSS and doses of neuroleptic medication and / or extrapyramidal side-effects.40-42 Hollander et al43 reported that NSS were more common in medication-free patients with OCD than in controls, while several studies found no influence of medication on NSS in OCD.12,20,21,23 In a meta- analysis, Jaafari et al44 found that medication effects were not associated with NSS.
Our study also aimed to find a correlation of NSS scores with age at onset in OCD patients. In patients with schizophrenia,45-48 higher degrees of focal motor signs with decreasing age at onset have been found, which is an inverse relationship between motor signs and age at onset in schizophrenic patients. However, no correlation was found between age at onset and NSS total score or the SI subscore in these studies. The authors hypothesised that 2 factors influence MC in patients with schizophrenia:
an early neurodevelopmental one and a later disease- dependent one, which correspond, respectively, to early- and late-onset schizophrenia.45-48 Several studies suggest that early-onset OCD is a different subgroup to late-onset OCD in phenotypic characteristics and could be associated with more brain anomalies.49,50 Thus, age at onset in OCD is a potential marker.
We tried to find a correlation between age at onset and NSS (NSS total scores and subscores) in OCD patients and found no correlation between these 2 variables. Our results are in line with the finding of Jaafari et al,25 who compared NSS in early- versus late-onset OCD (age < 13 years vs. ≥ 13 years), and found no significant difference in the NSS total scores or subscores. Two different definitions of age at onset in OCD have been given in the literature: (a) the age at which symptoms were first noticed by the individual and / or family member,49 and (b) the first time that symptoms altered functioning.50 In our study, we chose the former definition as it reflects the beginning of the disorder, whereas the latter defines the onset of the syndrome, so reflects the severity of the symptoms. Similar to our study, Jaafari et al25 had also taken the former definition of age at onset. However, we did not group the patients into early- and late-onset OCD as our study population were all adults (aged 18-55 years), but it would be useful to divide the patients into early- and late- onset OCD groups and then compare the NSS.
Fontenelle et al51 conducted a pilot study on 20 OCD patients, including 10 early-onset (mean age at onset, 11.1 years) and 10 late-onset OCD (mean age at onset, 41.7 years) patients, and evaluated NSS by a scale expanded by the authors and found that NSS are a frequent finding in OCD patients and do not seem to differ in terms of frequency between early- and late-onset OCD patients.
We found no correlation between the NSS scores and severity of disease (total YBOCS score) in the OCD group. These results are in line with several other studies.17,24,52,53 Jaafari et al44 did a meta-analysis and found that symptom severity of OCD was not associated with NSS. Although a study has found a positive correlation between the severity of obsession and increased levels of NSS, no correlation between disease severity (total YBOCS score) and NSS was noted.43 We did not group the OCD patients according to symptom type, which is a limitation of our study.
We had excluded psychosis in OCD, as this is a group that does not necessarily have psychosis and NSS are associated with the severity and persistence of psychopathological symptoms, particularly in schizophrenia.54 That may be a reason why we did not have higher rates of NSS in OCD patients than in controls.
Karadag et al55 compared OCD patients with poor insight and those with good insight, and healthy individuals, and demonstrated that OCD patients with poor insight exhibited significantly worse performance than healthy controls in all NSS categories and had more severe neurological findings in MC and SI subscales than OCD patients with good insight. Peng et al56 studied NSS in 100 patients with OCD, 38 patients with OCD and psychosis (22 with bipolar disorders and 16 with schizophrenia) as well as 101 healthy controls, and found that patients with OCD co-morbid with schizophrenia showed significantly higher scores in MC than patients with OCD and healthy controls.
The current thinking regarding the aetiopathogenesis of schizophrenia is that it may be a ‘progressive neurodevelopmental disorder’ with a developmental predisposition for early degeneration.57 According to this view, there is disruption in functional circuits involving heteromodal association areas rather than abnormality in a single brain region.58 These regions, including the frontal lobes, corpus callosum, basal ganglia, and recently, the cerebellum, have been extensively studied in patients with schizophrenia. Studies have pointed to a possible dysfunction in the cortical-thalamic-cerebellar-cortical circuit causing a ‘cognitive dysmetria’, which could explain the diversity of the disturbances in schizophrenia.59-61 There is significant evidence to suggest that NSS, including cerebellar signs, may form an intrinsic part of the syndrome of schizophrenia and lend strength to the neurodevelopmental hypothesis for the aetiopathogenesis of schizophrenia, as well as the model of cognitive dysmetria to explain some of the features seen in this enigmatic disorder.62
For OCD, the prevailing hypothesis suggests hyperactivity in the orbitofrontal cortex,50,63 medial caudate nucleus, thalamus, and anterior cingulate gyrus, with no argument supporting dysconnectivity as a feature. As we have found similar rates of NSS in OCD and controls and no correlation between NSS and age at onset, our findings do not support the hypothesis that NSS indicate neurodevelopmental brain anomalies associated with OCD, rather that NSS could be more specific to the neurodevelopmental anomalies associated with schizophrenia.
Thus, our study further strengthens the evidence regarding NSS in schizophrenia and supports the neurodevelopmental hypothesis of schizophrenia. Further studies using this information could be conducted to find the role of NSS in understanding the aetiology and prognosis of schizophrenia.
Whereas our study could not find higher rates of NSS in OCD, other neurological ‘subtle signs’, which are not present in these scales, could be worth exploring in OCD patients, in particular the signs assessing the fronto-striatal loops, cingulate cortex, and thalamic functions.
Limitations
Our study was limited by the small sample size, which restricts the generalisability of our results, and the study population might not have been representative of the sample. Influence of age and sex on NSS had not been taken into account. The effect of drugs and substance abuse had not been considered, which might confound the results. Although the instrument we used for NSS evaluation (CNI) is a standardised rating scale, it could potentially be confounded by the significant influence of subjectivity.
Conclusion
Neurological soft signs assessed by the CNI and YBOCS, which discriminate patients with schizophrenia from the healthy controls, appear to be relatively specific to schizophrenia. Further studies are required to explore NSS in OCD patients.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Diseases (DSM-IV). 4th ed. Washington DC: American Psychiatric Press; 1994.
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 2010;15:53-63.
- Hollander E, Liebowitz MR, Rosen WG. Neuropsychiatric and neuropsychological studies in obsessive-compulsive disorder. In: Zohar J, Insel T, Rasmussen S, editors. Psychobiology of obsessive- compulsive disorder. New York: Springer-Verlag; 1991: 126-45.
- Rapoport JL. Obsessive compulsive disorder and basal ganglia dysfunction. Psychol Med 1990;20:465-9.
- Rauch SL, Jenike MA. Neurobiological models of obsessive- compulsive disorder. Psychosomatics 1993;34:20-32.
- Rosenberg DR, Keshavan MS. A.E. Bennett Research Award. Toward a neurodevelopmental model of obsessive-compulsive disorder. Biol Psychiatry 1998;43:623-40.
- Winslow JT, Insel TR. Neuroethological models of obsessive compulsive disorder. In: Zohar J, Insel TR, Rasmussen S, editors. The psychobiology of obsessive-compulsive disorder. New York: Springer; 1991: 208-26.
- Murphy DL, Timpano KR, Wheaton MG, Greenberg BD, Miguel EC. Obsessive- compulsive disorder and its related disorders: a reappraisal of obsessive-compulsive spectrum concepts. Dialogues Clin Neurosci 2010;12:131-48.
- Saxena S, Rauch SL. Functional neuroimaging and the neuroanatomy of obsessive-compulsive disorder. Psychiatr Clin North Am 2000;23:563-86.
- Denckla MB. Neurological examination. In: Rapoport JL, editor. Obsessive-compulsive disorder in children and adolescents. Washington: American Psychiatric Press; 1989: 107-15.
- Hollander E, Schiffman E, Cohen B, Rivera-Stein MA, Rosen W, Gorman JM, et al. Signs of central nervous system dysfunction in obsessive-compulsive disorder. Arch Gen Psychiatry 1990;47:27-32.
- Hymas N, Lees A, Bolton D, Epps K, Head D. The neurology of obsessional slowness. Brain 1991;14:2203-33.
- Khanna S. Soft neurological signs in OCD. Biol Psychiatry 1991;29:442S.
- Schilder P. The organic background of obsessions and compulsions. Am J Psychiatry 1938;94:1397-420.
- Whitty PF, Owoeye O, Waddington JL. Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull 2009;35:415-24.
- Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull 2010;36:1089- 104.
- Sevincok L, Akoglu A, Arslantas H. Schizo-obsessive and obsessive- compulsive disorder: comparison of clinical characteristics and neurological soft signs. Psychiatry Res 2006;145:241-8.
- Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988;145:11-8.
- Conde López V, de la Gándara Martín JJ, Blanco Lozano ML, Cerezo Rodríguez P, Martínez Roig M, de Dios Francos A. Minor neurological signs in obsessive-compulsive disorders [in Spanish]. Actas Luso Esp Neurol Psiquiatr Cienc Afines 1990;18:143-64.
- Bihari K, Pato MT, Hill JL, Murphy DL. Neurologic soft signs in obsessive-compulsive disorder. Arch Gen Psychiatry 1991;48:278-9.
- Salama HM, Saad Allah HM, Mohamed NA. Study of the neurological soft signs in a sample of obsessive compulsive patients and its correlation with the severity of obsessive-compulsive symptoms and the degree of insight. Alex Bull 2008;44:9-12.
- Mataix-Cols D, Alonso P, Hernández R, Deckersbach T, Savage CR, Manuel Menchón J, et al. Relation of neurological soft signs to nonverbal memory performance in obsessive-compulsive disorder. J Clin Exp Neuropsychol 2003;25:842-51.
- Bolton D, Gibb W, Lees A, Raven P, Gray JA, Chen E, et al. Neurological soft signs in obsessive compulsive disorder: standardised assessment and comparison with schizophrenia. Behav Neurol 1998;11:197-204.
- Guz H, Aygun D. Neurological soft signs in obsessive-compulsive disorder. Neurol India 2004;52:72-5.
- Jaafari N, Baup N, Bourdel MC, Olié JP, Rotge JY, Wassouf I, et al. Neurological soft signs in OCD patients with early age at onset, versus patients with schizophrenia and healthy subjects. J Neuropsychiatry Clin Neurosci 2011;23:409-16.
- Stein DJ, Hollander E, Simeon D, Cohen L, Islam MN, Aronowitz B. Neurological soft signs in female trichotillomania patients, obsessive-compulsive disorder patients, and healthy control subjects. J Neuropsychiatry Clin Neurosci 1994;6:184-7.
- Flament MF, Whitaker A, Rapoport JL, Davies M, Berg CZ, Kalikow K, et al. Obsessive compulsive disorder in adolescence: an epidemiological study. J Am Acad Child Adolesc Psychiatry 1988;27:764-71.
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry 1981;38:381-9.
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46:1006- 11.
- Chen EY, Shapleske J, Luque R, McKenna PJ, Hodges JR, Calloway SP, et al. The Cambridge Neurological Inventory: a clinical instrument for assessment of soft neurological signs in psychiatric patients. Psychiat Res 1995;56:183-204.
- Holden EW, Tarnowski KJ, Prinz RJ. Reliability of neurological soft signs in children: reevaluation of the PANESS. J Abnorm Child Psychol 1982;10:163-72.
- Gupta S, Andreasen NC, Arndt S, Flaum M, Schultz SK, Hubbard WC, et al. Neurological soft signs in neuroleptic-naive and neuroleptic- treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry 1995;152:191-6.
- Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1989;27:335-50.
- Mohr F, Hubmann W, Cohen R, Bender W, Haslacher C, Hönicke S, et al. Neurological soft signs in schizophrenia: assessment and correlates. Eur Arch Psychiatry Clin Neurosci 1996;246:240-8.
- Rochford JM, Detre T, Tucker GJ, Harrow M. Neuropsychological impairments in functional psychiatric diseases. Arch Gen Psychiatry 1970;22:114-9.
- Lawrie SM, Byrne M, Miller P, Hodges A, Clafferty RA, Cunningham Owens DG, et al. Neurodevelopmental indices and the development of psychotic symptoms in subjects at high risk of schizophrenia. Br J Psychiatry 2001;178:524-30.
- Kolakowska T, Williams AO, Jambor K, Ardern M. Schizophrenia with good and poor outcome. III: Neurological ‘soft’ signs, cognitive impairment and their clinical significance. Br J Psychiatry 1985;146:348-57.
- Browne S, Clarke M, Gervin M, Lane A, Waddington JL, Larkin C, et al. Determinants of neurological dysfunction in first episode schizophrenia. Psychol Med 2000;30:1433-41.
- Schröder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M, et al. Neurological soft signs in schizophrenia. Schizophr Res 1991;6:25- 30.
- Quitkin F, Rifkin A, Klein DF. Neurologic soft signs in schizophrenia and character disorders. Organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch Gen Psychiatry 1976;33:845-53.
- Youssef HA, Waddington JL. Primitive (developmental) reflexes and diffuse cerebral dysfunction in schizophrenia and bipolar affective disorder: overrepresentation in patients with tardive dyskinesia. Biol Psychiatry 1988;23:791-6.
- King DJ, Wilson A, Cooper SJ, Waddington JL. The clinical correlates of neurological soft signs in chronic schizophrenia. Br J Psychiatry 1991;158:770-5.
- Hollander E, Kaplan A, Schmeidler J, Yang H, Li D, Koran LM, et al. Neurological soft signs as predictors of treatment response to selective serotonin reuptake inhibitors in obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci 2005;17:472-7.
- Jaafari N, Fernández de la Cruz L, Grau M, Knowles E, Radua J, Wooderson S, et al. Neurological soft signs in obsessive-compulsive disorder: two empirical studies and meta-analysis. Psychol Med 2013;43:1069-79.
- Owens DG, Johnstone EC, Frith CD. Spontaneous involuntary disorders of movement: their prevalence, severity, and distribution in chronic schizophrenics with and without treatment with neuroleptics. Arch Gen Psychiatry 1982;39:452-61.
- Björck RV, Själin M, Nordin C. Neurological soft signs in schizophrenic patients: influence of age, age at onset, sex, and family history of schizophrenia. Nord J Psychiatry 2000;54:437-40.
- Flashman LA, Flaum M, Gupta S, Andreasen NC. Soft signs and neuropsychological performance in schizophrenia. Am J Psychiatry 1996;153:526-32.
- Woods BT, Kinney DK, Yurgelun-Todd D. Neurologic abnormalities in schizophrenic patients and their families. I. Comparison of schizophrenic, bipolar, and substance abuse patients and normal controls. Arch Gen Psychiatry 1986;43:657-63.
- Jaisoorya TS, Janardhan Reddy YC, Srinath S. Is juvenile obsessive- compulsive disorder a developmental subtype of the disorder? Findings from an Indian study. Eur Child Adolesc Psychiatry 2003;12:290-7.
- Geller D, Biederman J, Jones J, Park K, Schwartz S, Shapiro S, et al. Is juvenile obsessive-compulsive disorder a developmental subtype of the disorder? A review of the pediatric literature. J Am Acad Child Adolesc Psychiatry 1998;37:420-7.
- Fontenelle L, Marques C, Engelhardt E, Versiani M. Soft neurological signs in early and late-onset obsessive-compulsive disorder: pilot study [in Spanish]. Psiquiatr Biol 2000;8:41-7.
- Sobin C, Blundell ML, Karayiorgou M. Phenotypic differences in early- and late-onset obsessive-compulsive disorder. Compr Psychiatry 2000;41:373-9.
- Poyurovsky M, Hramenkov S, Isakov V, Rauchverger B, Modai I, Schneidman M, et al. Obsessive-compulsive disorder in hospitalized patients with chronic schizophrenia. Psychiatry Res 2001;102:49-57.
- Jahn T, Hubmann W, Karr M, Mohr F, Schlenker R, Heidenreich T, et al. Motoric neurological soft signs and psychopathological symptoms in schizophrenic psychoses. Psychiatry Res 2006;142:191-9.
- Karadag F, Tumkaya S, Kırtaş D, Efe M, Alacam H, Oguzhanoglu NK. Neurological soft signs in obsessive compulsive disorder with good and poor insight. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1074-9.
- Peng ZW, Xu T, Miao GD, He QH, Zhao Q, Dazzan P, et al. Neurological soft signs in obsessive-compulsive disorder: the effect of co-morbid psychosis and evidence for familiality. Prog Neuropsychopharmacol Biol Psychiatry 2012;39:200-5.
- Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry 1998;155:1661-70.
- Lewis DA. Schizophrenia and disordered neural circuitry. Schizophr Bull 1997;23:529-31.
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A 1996;93:9985-90.
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical- subcortical-cerebellar circuitry? Schizophr Bull 1998;24:203-18.
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999;46:908-20.
- Varambally S, Venkatasubramanian G, Gangadhar BN. Neurological soft signs in schizophrenia — the past, the present and the future. Indian J Psychiatry 2012;54:73-80.
- Picard HJ, Amado I, Bourdel MC, Landgraf S, Olié JP, Krebs MO. Correlates between neurological soft signs and saccadic parameters in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:676-81.