East Asian Arch Psychiatry 2023;33:126-30 | https://doi.org/10.12809/eaap2340
CASE REPORT

Yolanda Yuen Ching Tsang, Nga Yu Hui, Sherry Kit Wa Chan
Abstract
We present a young woman with clozapine-resistant schizoaffective disorder who was treated with maintenance electroconvulsive therapy and multiple antipsychotics but continued to have auditory hallucinations. She had a haemorrhagic stroke secondary to a ruptured arteriovenous malformation at the right superior temporal gyrus, which was excised during emergency craniotomy. Despite having neurological deficits after the stroke, she reported cessation of auditory hallucinations. Magnetic resonance imaging of the brain showed Wallerian degeneration over the right temporal region. Personalised neuromodulation intervention may be a more effective treatment option for clozapine- resistant schizophrenia.
Yolanda Yuen Ching Tsang, Queen Mary Hospital, Hong Kong SAR, China
Nga Yu Hui, Queen Mary Hospital, Hong Kong SAR, China
Sherry Kit Wa Chan, Department of Psychiatry, Li Ka Shing Faculty of Medicine & The State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong SAR, China
Address for correspondence: Dr Sherry Kit Wa Chan, Room 219, New Clinical Building, Department of Psychiatry, The University of Hong Kong, Queen Mary Hospital, 102 Pokfulam Road, Hong Kong SAR, China. Email: kwsherry@gmail.com
Submitted: 27 July 2023; Accepted: 17 October 2023
Introduction
About 15% to 30% of patients with schizophrenia-spectrum disorders who do not experience satisfactory symptom improvement with antipsychotic medications are considered to have treatment-resistant schizophrenia.1 Clozapine is typically the only effective medication for these patients,2 and those who still do not experience satisfactory symptom improvement are considered to have clozapine-resistant schizophrenia.3 There is a lack of evidence to guide treatment of clozapine-resistant schizophrenia; electroconvulsive therapy (ECT) is one treatment option.4 In patients with clozapine-resistant schizophrenia who responded to ECT, 64% relapsed at 24 weeks; thus, maintenance ECT (M-ECT) can be used to prevent relapse.5 There is no clear guidance on therapies for those who do not respond to ECT. We present a patient with schizoaffective disorder who experienced intractable auditory hallucinations despite treatment with M-ECT, clozapine, and adjunctive antipsychotics.
Case Presentation
In 2012, a young woman with normal neurodevelopment and without family history of mental illness was admitted to our hospital because of referential delusions, depressed mood, mood-congruent auditory hallucinations, and a suicide attempt by jumping from height. The results of extensive blood work, electroencephalography, and radiological imaging were unremarkable. She was diagnosed with schizoaffective disorder and prescribed with risperidone up to 6 mg/day, but her symptoms persisted despite continuous treatment for over 4 weeks and even after switching to olanzapine 25 mg/day. She continued to experience persistent auditory hallucinations and depressed mood and made multiple suicide attempts in the ward. In view of her high suicide risk, she underwent 22 sessions of bilateral ECT, with improvement in psychotic and mood symptoms. Clozapine was initiated, and her psychotic symptoms subsided on clozapine 500 mg/day and sertraline 200 mg/day.
After discharge, she reported transient derogatory auditory hallucinations and commanding auditory hallucinations asking her to die. The voices were exacerbated by stress. From April to September 2014, she underwent further 18 sessions of ECT, and the dosage of clozapine was increased to 600 mg/day; clozapine was augmented with sodium valproate 500 mg/day. However, she still had frequent self-harm behaviours as she was distressed by the auditory hallucinations. From 2015 to 2021, she was commenced on M-ECT varying from twice weekly to once every 6 weeks. She continued to take clozapine 600 mg/day, together with amisulpride 700 mg/day and fluoxetine 60 mg/day. Despite experiencing auditory hallucinations on a daily basis and developing referential and persecutory beliefs that strangers might kill her, the symptoms were tolerable, and she was able to sustain work as a waitress.
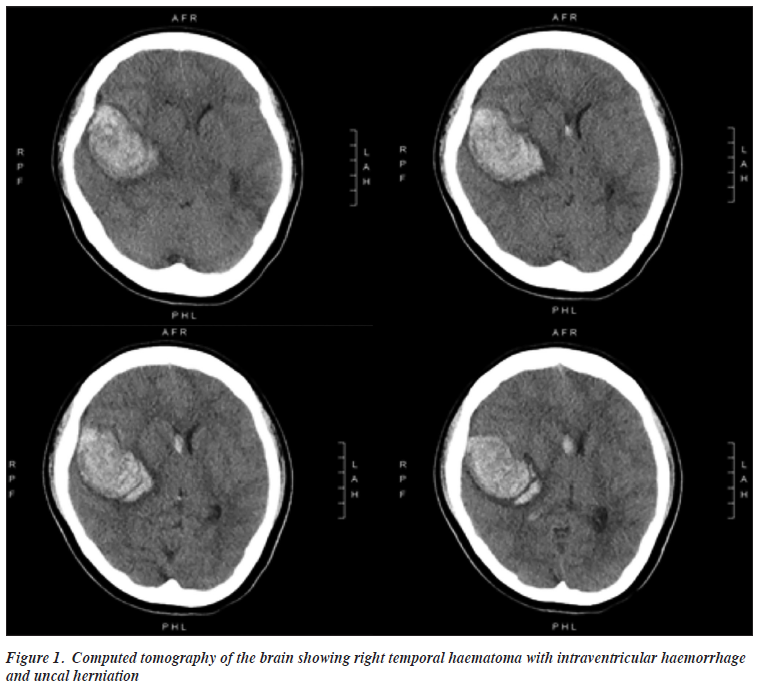
In March 2021, she was admitted to the neurosurgical ward because of a sudden loss of consciousness. Computed tomography of the brain showed a large right temporal haematoma with intraventricular haemorrhage and uncal herniation (Figure 1). Emergency craniotomy was performed, confirming a diagnosis of haemorrhagic stroke secondary to a ruptured arteriovenous malformation near the right superior temporal gyrus (STG), which was excised during the operation. Three weeks after the surgery, she was restarted on the same dose of clozapine and amisulpride but not the antidepressant (in view of the risk of haemorrhage).
She reported minimal auditory hallucinations before restarting the regimen, and she had complete remission of her psychotic symptoms 1 month after restarting the regimen. No further ECT was needed.
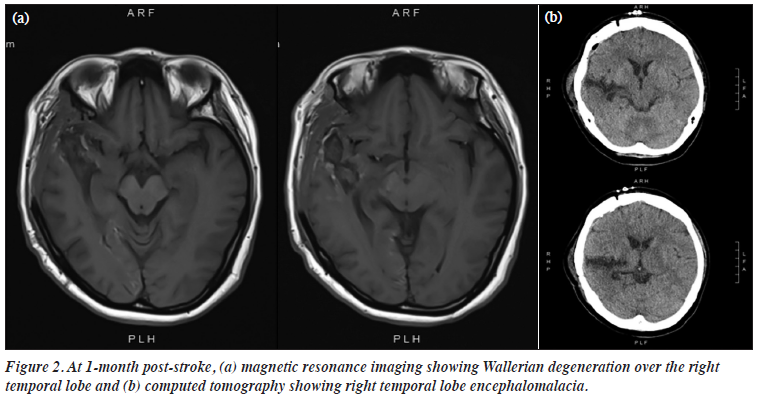
Her cognitive function improved with inpatient rehabilitation. Neuropsychological assessment on 23 April 2021 showed impairments in visual attention (score 19, Z = -7.64, <0.01th percentile), processing speed (score 9, Z = -7.40, <0.01th percentile), and working memory (score 3, Z = -2.63, 0.43th percentile), and apparent left visual neglect. Reassessment on 23 July 2021 showed persistent impairment in visual attention (score 19, Z = -7.64, <0.01th percentile) and processing speed (score 12, Z = -6.99, <0.01th percentile) but some improvement in working memory (score 4, Z = -1.81, 4th percentile). She continued to exhibit significant neurological deficits with left hemiparesis, left hemi-neglect, dysarthria, and mild dysphagia. At 1-month post-stroke, magnetic resonance imaging and computed tomography of the brain showed Wallerian degeneration and encephalomalacia, respectively, over the right temporal region (Figure 2). She remained free from psychotic and mood symptoms and was discharged in August 2021. Between August 2021 and December 2021, she reported no auditory hallucinations. She was intermittently distressed by her disability, but her mood remained stable. The dosage of amisulpride was gradually reduced from 700 mg to 300 mg, and she remained symptom-free.
Discussion
Post-stroke psychotic symptoms have been reported. Most lesions are over the right hemisphere (in descending frequency): in the parietal lobe, frontal lobe, temporal lobe, and caudate nucleus.5 However, our patient had resolution of auditory hallucinations after a haemorrhagic stroke. Similarly, a 70-year-old man with a history of depression and persecutory delusions was reported to experience 2 days of euphoria with stable mood after an ischaemic stroke of the right temporo-parietal area.6 He had no psychotic symptoms at 6-month follow-up and did not require any psychotropics. Post-stroke mania is suggested to be related to lesions over the right hemispheric ventral limbic circuit.7
In our patient, the arteriovenous malformation was located over the right STG, which contains the primary auditory cortex—Heschl’s gyrus and planum temporale, which help to discriminate, interpret, and monitor auditory input internally and externally.8 Auditory hallucinations involve abnormalities in the neural circuits responsible for normal language and auditory processing, in which the STG plays a major role.9 The STG has wide connections with the areas implicated in schizophrenia, namely the limbic system, thalamus, and prefrontal cortex.10 Individuals with schizophrenia have significantly smaller STG volumes, especially on the left side.11 Increased activation of the STG towards internally generated auditory stimuli reduces activation towards external stimuli.12 Heightened activity in the primary auditory cortex and areas responsible for language is noted when hallucinations are experienced.9 Disruptions in the STG of the non-dominant right hemisphere may explain the resolution of auditory hallucinations in our patient, although most evidence is associated with the left STG.13 In addition, the dorsolateral prefrontal cortex is associated with reduced volume in patients with psychosis, suggesting the involvement of frontotemporal interactions. Reduced functional frontotemporal connectivity has been reported in patients with clozapine-resistant schizophrenia.14 This supports the hypothesis that a lack of connectivity between the brain regions responsible for initiating acts and perceiving sensory consequences causes misattribution of internally generated stimuli to external origins, thereby leading to hallucinations.9 Aberrant connectivity between the prefrontal, temporal, anterior cingulate regions and structures in the medial temporal lobe (the amygdala, hippocampus, and parahippocampal gyrus) is implicated in the generation of psychotic symptoms.15
Invasive neurosurgical interventions such as leucotomy had been used to treat patients with schizophrenia.16 The negative consequences and inconsistent outcomes of neurosurgery and the effectiveness of chlorpromazine in controlling psychotic symptoms led to the discontinuation of neurosurgery in favour of antipsychotic medications. However, antipsychotics can cause distressing adverse effects, and some patients are non-responsive to antipsychotic treatment.
ECT is the oldest form of neuromodulation intervention. There are no contraindications to ECT, but care should be taken in patients with vascular malformations owing to the risk of bleeding and hence increased morbidity and mortality.17 Modulation of neuroplasticity and neurotrophic factors through ECT is associated with improved psychiatric symptoms.18 However, ECT does not target specific neurocircuitry. Our patient still experienced residual auditory hallucinations despite M-ECT. More targeted neuromodulation interventions may be needed. Deep brain stimulation, transcranial magnetic stimulation, and transcranial direct current stimulation may be more effective treatment options for schizophrenia. There is no consensus regarding the optimal placement of electrode for deep brain stimulation. Two of three patients with electrodes placed at the nucleus accumbens and two of four patients with electrodes placed at the subgenual anterior cingulate cortex had 25% improvement in their Positive and Negative Symptoms Scale total scores.19 Results from studies of transcranial magnetic stimulation and transcranial direct current stimulation are inconsistent. In patients with treatment-resistant schizophrenia, repetitive transcranial magnetic stimulation to left temporoparietal area at 1 Hz was reported to reduce auditory hallucinations significantly.20 Thus, personalised neuromodulation interventions according to the affected brain region are recommended. Further large-scale trials of personalised neuromodulation intervention are needed to determine optimal evidence-based treatment.
Contributors
All authors designed the study, acquired the data, analysed the data, drafted the manuscript, and critically revised the manuscript for important intellectual content. All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of Interest
As an editor of the journal, SKW Chan was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding / Support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
All data generated or analysed during the present study are available from the corresponding author on reasonable request.
Ethics approval
This study was approved by the Institutional Review Board of the University of Hong Kong and Hospital Authority Hong Kong West Cluster (UW22-294). The patient was treated in accordance with the tenets of the Declaration of Helsinki. The patient provided informed consent for all treatments and procedures and for publication.
References
- Chan SKW, Chan HYV, Honer WG, et al. Predictors of treatment- resistant and clozapine-resistant schizophrenia: a 12-year follow-up study of first-episode schizophrenia-spectrum disorders. Schizophr Bull 2021;47:485-94. Crossref
- Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2016;209:385-92. Crossref
- Campana M, Falkai P, Siskind D, Hasan A, Wagner E. Characteristics and definitions of ultra-treatment-resistant schizophrenia — A systematic review and meta-analysis. Schizophr Res 2021;228:218-26. Crossref
- Wagner E, Kane JM, Correll CU, et al. Clozapine combination and augmentation strategies in patients with schizophrenia: recommendations from an international expert survey among the Treatment Response and Resistance in Psychosis (TRRIP) Working Group. Schizophr Bull 2020;46:1459-70. Crossref
- Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry 2018;89:879-85. Crossref
- Chimowitz MI, Furlan AJ. Resolution of psychotic depression after right temporoparietal infarction. J Nerv Ment Dis 1990;178:458-9. Crossref
- Santos CO, Caeiro L, Ferro JM, Figueira ML. Mania and stroke: a systematic review. Cerebrovasc Dis 2011;32:11-21. Crossref
- McGuire PK, Silbersweig DA, Wright I, Murray RM, Frackowiak RS, Frith CD. The neural correlates of inner speech and auditory verbal imagery in schizophrenia: relationship to auditory verbal hallucinations. Br J Psychiatry 1996;169:148-59. Crossref
- Boksa P. On the neurobiology of hallucinations. J Psychiatry Neurosci 2009;34:260-2.
- Sumich A, Chitnis XA, Fannon DG, et al. Temporal lobe abnormalities in first-episode psychosis. Am J Psychiatry 2002;159:1232-5. Crossref
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry 2005;162:2233-45. Crossref
- Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia 2011;49:3361-9. Crossref
- Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res 2000;41:303-12. Crossref
- Pang TSW, Chun JSW, Wong TY, et al. A systematic review of neuroimaging studies of clozapine-resistant schizophrenia. Schizophrenia (Heidelb) 2023;9:65. Crossref
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res 2001;49:1-52. Crossref
- Agarwal P, Sarris CE, Herschman Y, Agarwal N, Mammis A. Schizophrenia and neurosurgery: a dark past with hope of a brighter future. J Clin Neurosci 2016;34:53-8. Crossref
- Mehdi SMA, Devanand DP. Electroconvulsive therapy in elderly patients with cerebral aneurysms: a systematic review with clinical recommendations. J Geriatric Psychiatry Neurol 2021;34:504-12. Crossref
- Wang J, Tang Y, Curtin A, et al. ECT-induced brain plasticity correlates with positive symptom improvement in schizophrenia by voxel-based morphometry analysis of grey matter. Brain Stimul 2019;12:319-28. Crossref
- Corripio I, Roldán A, Sarró S, et al. Deep brain stimulation in treatment resistant schizophrenia: a pilot randomized cross-over clinical trial. EBioMedicine 2020;51:102568. Crossref
- Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IE. Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry 2014;76:101-10. Crossref