East Asian Arch Psychiatry 2016;26:87-97
ORIGINAL ARTICLE
Dr Wei Zheng, MD, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, PR China.
Dr Yan-Fang Zhang, MD, The National Clinical Research Center for Mental Disorders, Beijing, China & Center of Depression, Beijing Institute for Brain Disorders, PR China; Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, PR China.
Dr Hua-Qing Zhong, MD, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, PR China.
Dr Si-Ming Mai, MD, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, PR China.
Dr Xin-Hu Yang, MD, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, PR China.
Prof. Yu-Tao Xiang, MD, PhD, Unit of Psychiatry, Faculty of Health Sciences, University of Macao, Macao SAR, PR China.
* The authors have contributed equally to this article.
Address for correspondence: Prof. Yu-Tao Xiang, 3/F, Building E12, Faculty of Health Sciences, University of Macau, Avenida da Universidade, Taipa, Macao SAR, PR China.
Tel: (853) 8822 4223; Fax: (853) 2288 2314; Email: xyutly@gmail.com
Submitted: 4 June 2016; Accepted: 19 July 2016
Abstract
Introduction: In China, Wuling capsule, a traditional Chinese medicine consisting of Wuling mycelia of Xylaria nigripes (Kl.) Sacc (a rare type of fungus), is used to treat major depressive disorders. A meta- analysis of randomised controlled trials was performed to compare the efficacy and safety of Wuling capsule alone with Wuling capsule–antidepressant combination in the treatment of major depressive disorders.
Methods: Two assessors independently selected studies, extracted data, and conducted quality assessment and data synthesis. Standard mean difference, risk ratio (RR) ± 95% confidence interval (CI), the number needed to treat, and the number needed to harm were analysed.
Results: A total of 12 randomised controlled trials (880 patients; mean age ± standard deviation, 39.7 ± 12.5 years; male patients, 41%) were identified, including 4 trials with Wuling capsule alone (n = 340) and 8 with Wuling capsule–antidepressant (sertraline, mianserin, mirtazapine, and paroxetine) combination (n = 540). The mean length of trial was 5.7 ± 1.3 weeks. Meta-analysis of symptomatic improvement at last-observation endpoint and study-defined response and remission revealed no significant differences between the Wuling capsule alone and antidepressant monotherapy. The Wuling capsule–antidepressant cotreatment was superior to antidepressant monotherapy in symptomatic improvement at last-observation endpoint (standard mean difference: -0.46, p = 0.001) as well as study-defined response (68.4% vs. 56.0%, RR = 1.23; p = 0.03) and remission (46.5% vs. 34.5%, RR = 1.35; p = 0.05). Wuling capsule was associated with fewer adverse drug reactions than antidepressant monotherapy.
Conclusions: Adjunctive Wuling capsule may augment the effects of antidepressants and may be associated with fewer adverse drug reactions. More large-scale and rigorously designed randomised controlled trials with large sample size are warranted to clarify the effectiveness of Wuling capsule for major depressive disorders.
Key words: Antidepressive agents; Depressive disorder, major; Drugs, Chinese herbal
Introduction
Major depressive disorder (MDD) is common and causes immense suffering for patients and their family, physical and mental disability, poor quality of life, and increased mortality.1 The World Health Organization predicts that MDD will be ranked the second most common cause of disease burden by the year 2020.2 In Chinese traditional medicine, ‘liver qi stagnation’ (肝氣鬱結), meaning unbalanced inner state, may contribute to MDD; correcting this unbalanced inner state may help improve depressive symptoms.3-5 Several systematic reviews have found that traditional Chinese medicines that act on inner balance are safe and effective in MDD.4,6
Wuling capsule, a traditional Chinese medicine, is comprised mainly of Wuling mycelia of Xylaria nigripes (Kl.) Sacc (a rare type of fungus). Wuling capsule can strengthen the immune system, improve anxiety and depressive symptoms, and has been approved to treat insomnia and depression by the China Food and Drug Administration (CFDA) [Authorized Document No.: Z19990048 in Chinese medicine] since 1998.5,7 According to the Chinese Pharmacopoeia (version 2005 and 2010), the ingredients and production of Wuling capsule are subject to strict quality control and standardisation.7 The CFDA recommends that Wuling capsule be given at a dose of 2970 mg a day (330 mg/tablet, 3 tablets, 3 times per day).
In China, Wuling capsule has been either used alone or as an adjunct to antidepressants (ADs) in the treatment of MDD. Results of randomised controlled trials (RCTs) have been inconsistent.8-19 There has been no systematic review or meta-analysis evaluating Wuling capsule in the treatment of MDD. We therefore carried out a systematic review and meta-analysis of RCTs to assess the efficacy and safety of Wuling capsule in treating MDD.
Methods
Types of Studies
Any RCTs in Chinese or English language that reported the effects of Wuling capsule in MDD were eligible for inclusion in this review. Case series, non-randomised and animal trials, and reviews were excluded.
Types of Participants
Patients with a diagnosis of MDD according to the DSM-IV,20 the ICD-10,21 or the third edition of Chinese Classification of Mental Disorders22 were included.
Types of Outcome Measures
The primary outcome was endpoint symptomatic improvement, based on intention-to-treat (ITT) analysis if provided, assessed by the Hamilton Depression Rating Scale23 (HAMD-17, HAMD-21 and HAMD-24), Zung Self-Rating Depression Scale,24 or Edinburgh Postnatal Depression Scale (EPDS).25 Key secondary outcomes included response and remission as defined by individual studies (apart from the diagnostic methods of traditional Chinese medicine), improvement in anxiety symptoms assessed by the Hamilton Anxiety Rating Scale (HAMA),26 rate of all-cause discontinuation, and patient-reported adverse events.
Data Search
English databases (PubMed, PsycINFO, Embase, Cochrane Library databases, the Cochrane Register of Controlled Trials) and Chinese databases (WanFang Database, Chinese Biomedical Databases, and China Journal Net) were searched from inception of the database until October 2015, using the following search terms: (‘depressive disorders’ or ‘disorder’, ‘depressive’ or ‘disorders’, ‘depressive’ or ‘neurosis’, ‘depressive’ or ‘depressive neuroses’ or ‘depressive neurosis’ or ‘neuroses’, ‘depressive’ or ‘depression’, ‘endogenous’ or ‘depressions’, ‘endogenous’ or ‘endogenous depression’ or ‘endogenous depressions’ or ‘depressive syndrome’ or ‘depressive syndromes’ or ‘syndrome’, ‘depressive’ or ‘syndromes’, ‘depressive’ or ‘depression’, ‘neurotic’ or ‘depressions’, ‘neurotic’ or ‘neurotic depression’ or ‘neurotic depressions’ or ‘melancholia’ or ‘melancholies’ or ‘unipolar depression’ or ‘depression’, ‘unipolar’ or ‘depressions’, ‘unipolar’ or ‘unipolar depressions’) and (‘Wuling’ or ‘Wu ling’ or ‘Wu- ling’). The search was supplemented using the “related article” function. Reference lists from identified studies were manually searched for additional studies, and authors of unpublished or missing data were contacted.
Data Extraction
Two of the authors (WZ and XHY) independently conducted the search, study selection, data extraction, quality assessment, and data synthesis. Any inconsistencies were resolved by discussion with another author (YTX).
Assessment of Study Quality
According to the recommendation of the Cochrane Collaboration,27 we assessed the methodological quality of each study using the risk of bias including the following terms: (1) random sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias), and (7) other bias. Furthermore, 5 domains of the Jadad scale, ‘randomisation’, ‘double blinding’, ‘description withdrawals and dropouts’, ‘generation of random numbers’, and ‘allocation concealment’ were rated on a scale of 0 to 5 to characterise study quality.28 The quality of RCTs was rated as ‘high’ when Jadad score was ≥ 3; otherwise it was rated as ‘low’. The quality of evidence and strength of recommendations of outcome measure of Wuling capsule for MDD was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system as ‘very low’, ‘low’, ‘moderate’, or ‘high’.29,30
Statistical Analyses
Statistical analyses were performed using the Review Manager (version 5.1.7.0). For continuous data, the effect size was determined by calculating standard mean difference (SMD) or weighted mean difference (WMD) with 95% confidence interval (CI); for dichotomous data risk ratio (RR) ± 95% CI was computed. The number needed to treat (NNT) or number needed to harm (NNH) was calculated if RR was significant by dividing 1 by the risk difference. Heterogeneity was examined with I2 statistic: if I2 ≥ 50%, a random effect model was used31; otherwise, a fixed effects model was employed. In case of a limited number of meta- analytic trials (< 10) in each outcome, publication bias was not assessed. All statistical differences were considered significant when p < 0.05.
When heterogeneity was present (I2 ≥ 50%), subgroup and meta-regression analyses were used to explore the reasons for heterogeneity.
Results
Results of the Search
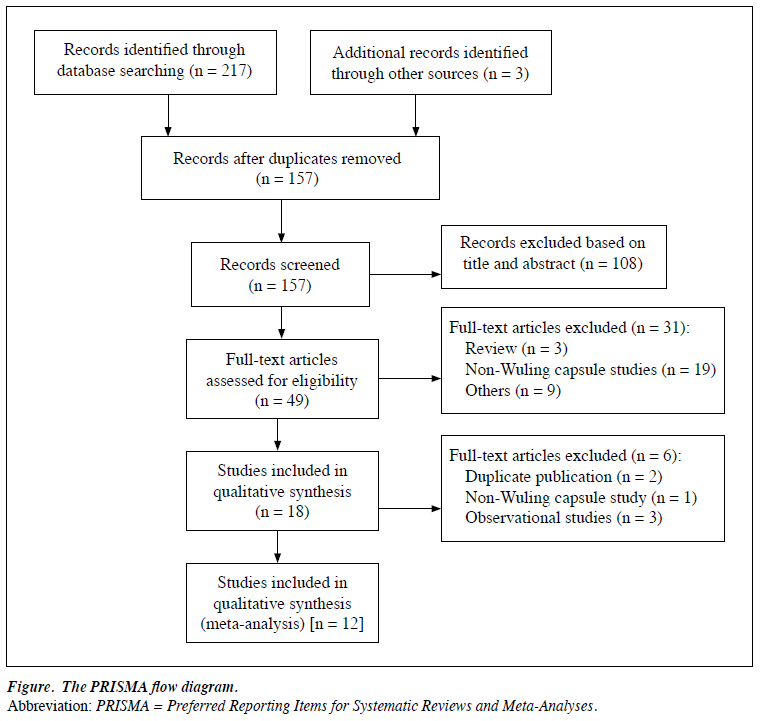
The systematic search yielded 220 potentially relevant articles from English (73 trials) and Chinese (147 trials) databases; 63 studies were shown by EndNote 7.1 software to be duplicates. After reviewing the title or abstract (108 trials) or full text (37 trials), 12 RCTs were eligible in the meta-analysis (Table 18-19 and Fig).
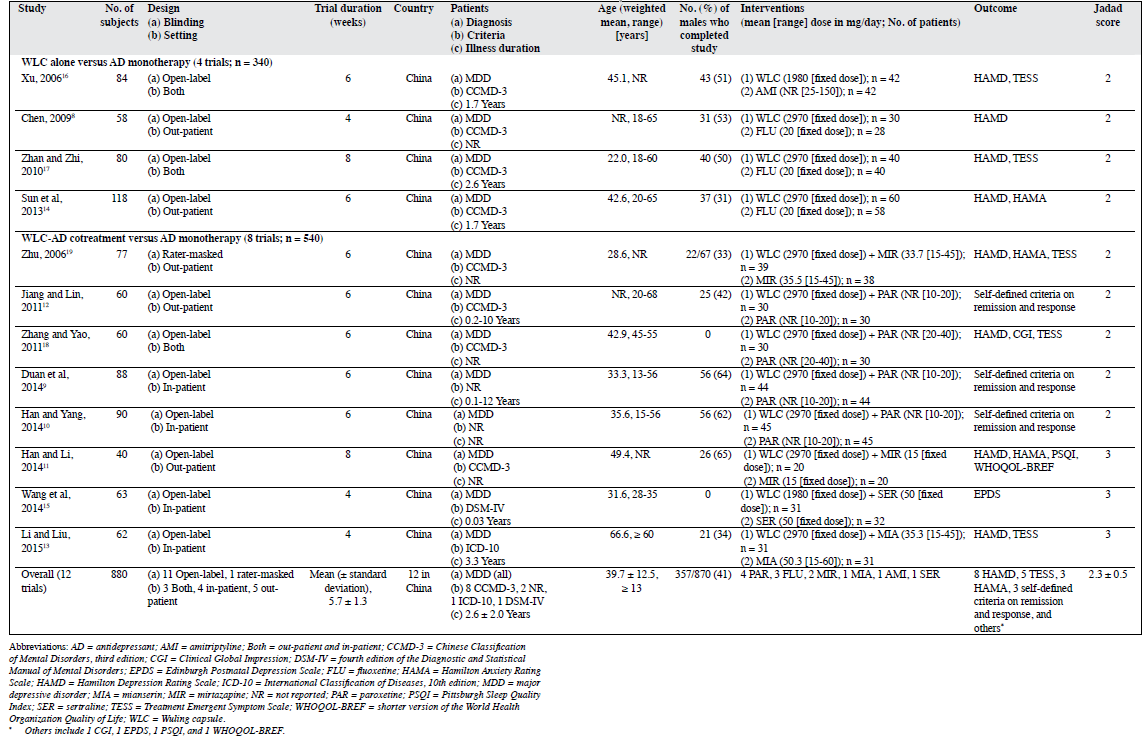
Characteristics of Studies
All RCTs were conducted in China. These 12 RCTs involved 880 patients with MDD (range, 40-118; median, 70 MDD patients). Of these, 340 were in the Wuling capsule–alone group and 540 were in the Wuling capsule–AD cotreatment group. The ADs used included amitriptyline, fluoxetine, mianserin, mirtazapine, sertraline, and paroxetine. The mean (± standard deviation) age of patients was 39.7 ± 12.5 years (mean range, 22.0-66.6 years; median, 39.1 years), and trial duration being 5.7 ± 1.3 weeks (range, 4-8 weeks; median, 6 weeks). The mean proportion of males was 41% (range, 0-65%; median, 46.0%), and the mean total illness duration was 2.6 ± 2.0 years (range, 0.03-12.0 years; median, 1.7 years). The mean dosage of Wuling capsule was 2805.0 ± 385.4 mg/day (range, 1980.0-2970.0 mg/day; median, 2970.0 mg/day).
Quality Assessment
Although “random” assignment with specific description was mentioned in 3 RCTs, most studies were rated as unclear risk (7 trials) or high risk (2 trials). Furthermore, most RCTs were rated as high risk regarding the allocation concealment methods (12 trials), masked assessors (12 trials), and blinding of the assessments (11 trials). Regarding outcome data, only 1 RCT reported lost to follow-up without using ITT analysis to deal with incomplete outcome data. Other bias was rated as low risk (12 trials), and selective reporting was rated as unclear risk in all RCTs that failed to employ a protocol registration (Appendix 18-19).

Study quality ranged from 2 to 3 on the Jadad scale score (mean, 2.3 ± 0.5; median, 2.0) [Table 18-19]. Three RCTs were rated as high quality and the remaining were of low quality. Based on the GRADE approach, the quality of evidence presented for each outcome ranged from ‘very low’ (9.1%), ‘low’ (72.7%), or ‘moderate’ (18.2%) [Table 2].

Wuling Capsule Alone Versus Antidepressant Monotherapy
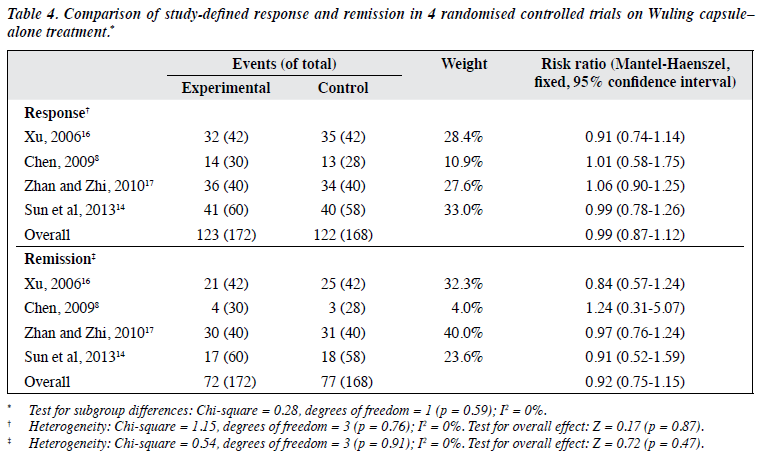
There was no significant difference between Wuling capsule alone and AD monotherapy regarding endpoint of HAMD total score in 4 RCTs (n = 340) with a SMD of 0.06 (95% CI, -0.15 to 0.28; p = 0.56; I2 = 0%) [Table 38,14,16,17]. The results were similar for study-defined response (71.5% vs. 72.6%; ≥ 50% reduction in HAMD total score in 4 trials) with a RR of 0.99 (95% CI, 0.87-1.12; p = 0.87; I2 = 0%) as well as remission (41.9% vs. 45.8%; reduction in HAMD total score ≥ 75% in 3 trials8,14,17 or ≥ 80% in 1 trial16) with a RR of 0.92 (95% CI, 0.75-1.15; p = 0.47; I2 = 0%) [Table 48,14,16,17]. One RCT14 found no advantage of Wuling capsule alone with regard to anxiety symptoms.

Wuling Capsule–antidepressant Cotreatment Versus Antidepressant Monotherapy
The Wuling capsule–AD cotreatment outperformed AD monotherapy regarding the endpoint HAMD total score in 4 RCTs (n = 229) with a SMD of -0.46 (95% CI, -0.73 to -0.20; p < 0.001; I2 = 5%) [Table 511,13,18,19]. Similar results were found for study-defined response (68.4% vs. 56.0%; reduction in HAMD total score ≥ 50% in 3 trials11,18,19 and EPDS total score ≥ 50% in 1 trial15) with a RR of 1.23 (95% CI, 1.03-1.47; p = 0.03; I2 = 44%; NNT = 8, 95% CI, 4-50), as well as remission (46.5% vs. 34.5%; reduction in HAMD total score ≥ 75% in 1 trial18 or ≥ 80% in 1 trial11, HAMD total score ≤ 7 in another trial19 and EPDS total score ≥ 75% in 1 trial15) with a RR of 1.35 (95% CI, 1.01-1.82; p = 0.05; I2 = 0%) [Table 611,15,18,19].


The Wuling capsule–AD cotreatment also outperformed AD monotherapy regarding the endpoint of HAMA total score in 2 RCTs (n = 107) with a WMD of -2.37 (95% CI, -3.59 to -1.14; p < 0.001; I2 = 0%) [Appendix 211,19]. Only 1 RCT11 that employed the Pittsburgh Sleep Quality Index and the World Health Organization Quality of Life–BREF focused on sleep quality and quality of life, respectively.

Subgroup and meta-regression analyses were not performed for endpoint symptomatic improvement, study- defined response or remission (Wuling capsule alone vs. Wuling capsule–AD cotreatment), since the heterogeneity was small (endpoint symptomatic improvement: I2 = 0% vs. 5%, study-defined response: I2 = 0% vs. 44%, study-defined remission: I2 = 0% vs. 0%).
Adverse Drug Reactions
The Treatment Emergent Symptom Scale was employed to assess adverse drug reactions (ADRs) in 5 RCTs.11,13,15,18,19
Wuling Capsule Alone Versus Antidepressant
Monotherapy
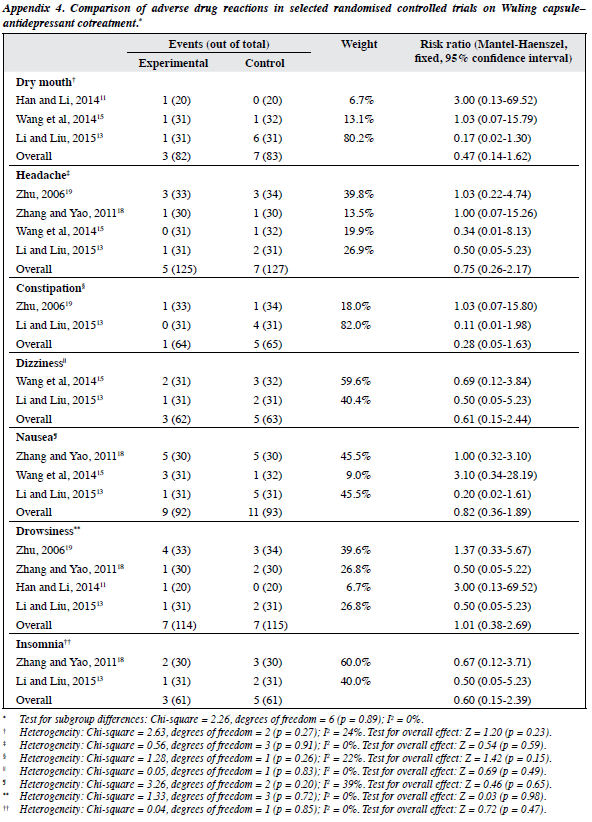
As shown in 2 RCTs (n = 164; Appendix 316,17), Wuling capsule was significantly associated with fewer ADRs than the control condition for nausea with a NNH of 6 (95% CI, 4-14; p = 0.01), for constipation with a NNH of 9 (95% CI = 6-33; p = 0.02), for tachycardia with a NNH of 9 (95% CI, 5-33; p = 0.02), and for dry mouth with a NNH of 6 (95% CI, 4-11; p = 0.003). No significant differences with respect to other ADRs were noted.
Wuling Capsule–antidepressant Cotreatment Versus Antidepressant Monotherapy
Meta-analysis of dry mouth, headache, constipation, dizziness, nausea, drowsiness, insomnia with a RR of 0.28 to 1.01 (95% CI, 0.05-2.69; p = 0.15-0.98; I2 = 0-39%) failed to find significant group differences (Appendix 411,13,15,18,19).
Discussion
Major Findings
This was the first meta-analysis of Wuling capsule in the treatment of MDD. The meta-analysis involved 12 RCTs totaling 880 MDD patients. The main finding of the study was that Wuling capsule alone and AD monotherapy appear to have similar efficacy in terms of endpoint HAMD score as well as response and remission rates. Further, Wuling capsule appears to have an augmenting effect on ADs in treating MDD. Both Wuling capsule alone and Wuling capsule–AD cotreatment were safe and well tolerated.
Wuling mycelia, the major component of Wuling capsule, contains amino acids, vitamins (B1, B2, B6, E, A, D2, and K1), microminerals, and micronutrients.5,7
The mechanisms of Wuling’s antidepressant effects as monotherapy or in augmenting ADs have been hypothesised to be improvement in the permeability of excitatory neurotransmitter Glu and vitamin B6 in the brain by Wuling mycelia that may increase activity of glutamate decarboxylase and the synthesis of gamma- aminobutyric acid and improve the activity of its receptor.5,7
The mechanism of Wuling’s action on anxiety and sleep in MDD is less understood.
Limitations
There are a number of limitations. First, according to the GRADE approach,29,30 9.1% and 72.7% quality of evidence was rated as ‘very low’ or ‘low’, respectively. Nonetheless, Guyatt et al32 suggested that strong recommendations do not necessarily imply high-quality evidence and low- quality evidence can still result in strong recommendations. Second, the small sample size (median, 70) in most studies might have produced false-negative results. Third, the publication bias test was not conducted due to the insufficient number of eligible studies for each outcome. Fourth, databases in a language other than English or Chinese were not searched and could constitute selection bias. Fifth, most studies were of short duration (median, 6 weeks), therefore the efficacy and safety of Wuling capsule for MDD beyond 8 weeks was not adequately studied. Finally, the benefit of Wuling capsule in enhancing sleep quality was examined in only 1 study,11 thus Wuling’s effect on sleep pattern could not be analysed.
Conclusions
This meta-analysis of 12 RCTs that involved 880 MDD patients indicated that Wuling is safe and has similar efficacy in treating MDD to standard ADs. Wuling may also augment the effects of mirtazapine, paroxetine, and mianserin, and is associated with fewer ADRs. More large- scale and rigorously designed RCTs with large sample size are warranted to clarify the effectiveness of Wuling capsule for MDD.
Appendices
Additional material related to this article can be found on the journal website. Please go to <http://www.easap.asia>, and search for the article.
Acknowledgements
The study was supported by the Start-up Research Grant (SRG2014-00019-FHS) and the Multi-Year Research Grant (MYRG2015-00230-FHS) from the University of Macao. We thank Prof. Gabor S. Ungvari in University of Western Australia, Prof. Helen F. K. Chiu in The Chinese University of Hong Kong, and Dr Chee H. Ng in University of Melbourne for their comments on the manuscript.
Declaration
The trial registration No. is CRD42015026430. The authors declare no conflicts of interest concerning this article.
References
- Bolton JM, Pagura J, Enns MW, Grant B, Sareen J. A population- based longitudinal study of risk factors for suicide attempts in major depressive disorder. J Psychiatr Res 2010;44:817-26.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498-504.
- Gao H, Shang YZ, Xia T, Qiao MQ, Zhang HY, Ma YX. The correlation between neurosteroids and neurotransmitters with liver yang rising and liver qi stagnation types of premenstrual syndrome. Gynecol Endocrinol 2014;30:913-7.
- Yeung WF, Chung KF, Ng KY, Yu YM, Zhang SP, Ng BF, et al. Prescription of Chinese herbal medicine in pattern-based traditional Chinese medicine treatment for depression: a systematic review. Evid Based Complement Alternat Med 2015;2015:160189.
- Peng L, Zhang X, Kang DY, Liu XT, Hong Q. Effectiveness and safety of Wuling capsule for post stroke depression: a systematic review. Complement Ther Med 2014;22:549-66.
- Yeung WF, Chung KF, Ng KY, Yu YM, Ziea ET, Ng BF. A systematic review on the efficacy, safety and types of Chinese herbal medicine for depression. J Psychiatr Res 2014;57:165-75.
- Lin Y, Wang XY, Ye R, Hu WH, Sun SC, Jiao HJ, et al. Efficacy and safety of Wuling capsule, a single herbal formula, in Chinese subjects with insomnia: a multicenter, randomized, double-blind, placebo- controlled trial. J Ethnopharmacol 2013;145:320-7.
- Chen K. A clinical study of Wuling capsule in the treatment of major depressive disorder [in Chinese]. Guide China Med 2009;7(14):51-2.
- Duan XC, Yuan MJ, Zhang ZR. A controlled trial of Wuling capsule combined with paroxetine for major depressive disorder [in Chinese]. Asia Pac Tradit Med 2014;(5):113-4.
- Han K, Yang JQ. Clinical observation on treating depression with Wuling capsule plus aroxetine [in Chinese]. Clin J Chin Med 2014;6(34):64-5.
- Han XQ, Li HH. Clinical efficacy of Wuling capsule combined with mirtazapine in the treatment of major depressive disorder [in Chinese]. Hunan J Tradit Chin Med 2014;(10):47-9.
- Jiang HC, Lin H. A controlled trial of Wuling capsule combined with paroxetine in the treatment of major depressive disorder [in Chinese]. Mod Tradit Chin Med 2011;(5):32-3.
- Li RZ, Liu CX. A clinical study of Wuling capsule combined with mianserin for old patients with major depressive disorder [in Chinese]. Chin J Mod Drug Appl 2015;(17):118-9.
- Sun L, Gu CH, Ren JX, Tian Y, Yang LB, Zhang XF. A randomized controlled study of Wuling capsule in the treatment of major depressive disorder [in Chinese]. Chin J Basic Med Tradit Chin Med 2013;(3):290-1.
- Wang Z, Chen L, Zuo QX, Xu ZM, Mo YL, Chen ZY. A research of Wuling capsule combined with sertraline in the treatment of postpartum depression and the change of hormone and neuro- transmitter in vivo [in Chinese]. J Zhejiang Univ Tradit Chin Med 2014;(3):250-4.
- Xu YS. A paralleled comparative study of Wuling capsule and amitriptyline in the treatment of mild depression [in Chinese]. J Shandong Med Coll 2006;(4):281-2.
- Zhan DH, Zhi SL. A clinical study of Wuling capsule for major depressive disorder [in Chinese]. Her Med 2010;(8):1032-3.
- Zhang CH, Yao WB. The efficacy and safety of Wuling capsule combined with paroxetine in the treatment of postpartum depression [in Chinese]. Seek Med Ask the Med 2011;9(12):545-6.
- Zhu YP. Comparison of mirtazapine united with Wuling capsule and mirtazapine alone for depression and anxiety comorbidity [in Chinese]. Pract Clin J Integr Tradit Chin West Med 2006;(6):5-6.
- Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington: American Psychiatric Association; 1994.
- World Health Organization. The International Classification of Diseases, tenth revision. Geneva, World Health Organization; 1992.
- Chen YF. Chinese classification of mental disorders (CCMD-3): towards integration in international classification. Psychopathology 2002;35:171-5.
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62.
- Zung WW. A self-rating depression scale. Archives of General Psychiatry 1965;12:63-70.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6.
- Snaith RP, Baugh SJ, Clayden AD, Husain A, Sipple MA. The Clinical Anxiety Scale: An Instrument Derived from the Hamilton Anxiety Scale. Br J Psychiatry 1982;141:518-23.
- Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2008.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1- 12.
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401-6.
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta- analysis. Stat Med 2002;21:1539-58.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso- Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.