Hong Kong J Psychiatry 2002;12(4):23-27
REVIEW ARTICLE
VWC Liu, LW Lam, HFK Chiu
Victor WC Lui, MRCPsych(UK), Medical Officer, Prince of Wales Hospital, Shatin, Hong Kong, China.
Linda CW Lam, MRCPsych(UK), FHKCPsych, FHKAM(Psy), Associate Professor, Department of Psychiatry, The Chinese University of Hong Kong, Hong Kong, China.
Helen FK Chiu, FRCPsych(UK), FHKCPsych, FHKAM(Psy), Professor, Department of Psychiatry, The Chinese University of Hong Kong, Hong Kong, China.
Address for correspondence: Lui Wing Cheong, Department of Psychiatry, Prince of Wales Hospital, Shatin, Hong Kong, China.
E-mail: victorluiwc@netvigator.com
Submitted: 18 September 2002; Accepted: 28 December 2002
Abstract
Tricyclic antidepressants have been the mainstay treatment modality of neuropathic pain. However, use of these agents has been limited by their significant side effects and potential cardiac adverse events, particularly in overdose. Newer antidepressants have been shown to have better safety profiles and tolerability. This article examines current evidence of the use of newer antidepressants in the treatment of neuropathic pain. The proposed mechanisms of action, clinical evidence and tolerability were reviewed. Although tricyclic antidepressants appear to be the most efficacious agents for neuropathic pain, there is inadequate evidence supporting their prolonged use. Paroxetine, trazodone, nefazodone, mirtazapine, and venlafaxine are potentially useful agents, but these drugs can also produce treatment-limiting side effects.
Key words: Antidepressive agents, Diabetic neuropathies, Neuralgia
Introduction
Neuropathic pain is the pain initiated or caused by a primary lesion or dysfunction in the nervous system.1,2 The possible aetiologies could be central in cases of stroke, mul- tiple sclerosis, or epilepsy, or peripheral in conditions such as diabetic neuropathy, postherpetic neuralgia, and traumatic nerve injury.2,3 There is no pathognomonic description for neuropathic pain in the literature, but the combination of burning or electrical pain with numbness and tingling, as well as pins and needles are thought to be highly indicative.2
Since this pain is usually chronic and responds poorly to analgesics, many non-pharmacological and pharmacological treatments have been proposed. For example, transcutaneous electrical nerve stimulation (TENS) had been shown to be effective in the treatment of diabetic neuropathy.4
A large variety of pharmacological agents, topical or oral, are also being used. These include antidepressants (especially the tricyclic antidepressants; TCAs), anti- convulsants, gamma-aminobutyric acid (GABA)-B receptor agonists, N-methyl-D-aspartate (NMDA) antagonists, opioids, levodopa and topical capsaicin creams.
According to the systematic review by Sindrup and Jensen, TCAs are the most efficacious agents for the treatment of neuropathic pain.5 While newer antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and venlafaxine share some similar pharmacodynamic properties with TCAs, the former may be potentially useful for the treatment of neuropathic pain, especially for patients with comorbid depression and suicide risk. Newer antidepressants have additional benefits — they are better tolerated and safer in overdose.6 While the side effects of TCAs may limit the achievement of optimal dose, this problem is less likely with newer antidepressants. In this review, the current evidence for possible underlying mechanisms and side effect profiles of antidepressants commonly used in the treatment of neuropathic pain are examined.
Tricyclic Antidepressants
The efficacy of TCAs in the treatment of depression was originally reported by Kuhn in 1958.7 Thereafter, TCAs came to be considered the mainstay treatment for depression. Although monoamine reuptake blockade properties seem to be the important initial mediator for their antidepressant action, the main mechanism is thought to be a delayed adaptive downregulation of postsynaptic receptors.8
The use of TCAs in the treatment of neuropathic pain can be dated back to a report by Woodforde et al of treatment of postherpetic neuralgia in 1965.9 However, early studies of tricyclic antidepressants were complicated by the concomitant use of other drugs.10 In 1982, Watson et al reported one of the earliest randomised, double-blind, placebo-controlled, crossover trials studying the effects of amitriptyline in the treatment of postherpetic neuralgia.10 The study recruited 24 patients and employed a treatment duration of 3 weeks. The effects on depression, sleep, and untoward reactions were monitored. The drug relieved pain for most of the non-depressed patients (11 of 14) and showed good pain-relieving response for 1 patient whose depression persisted. Among patients with good pain relief, the blood levels of amitriptyline and nortriptyline were below or at the lower end of the therapeutic ranges usually associated with antidepressant effects.
In 1984, Kvinesdal et al reported the efficacy of imipramine in painful diabetic neuropathy.11 In this fixed- dose, double-blind, randomised, placebo-controlled, cross- over study, the drug regimen was 50 mg/day during the first week and 100 mg/day from the second to the fifth week. Seven patients showed improvement in the global assessment while taking imipramine and none had improvement in the placebo period. It was reported that most of the beneficial effects occurred after 1 week of treatment. During the past 20 years, amitriptyline,10,12-14 desipramine,12,15-16 imipramine11, nortriptyline,17 and clomipramine18 were shown to be useful in relieving neuropathic pain in various placebo-controlled trials. In each case, the efficacy appeared to be independent of the character of the pain19 and the drug effect on mood.20 As for the possible mechanisms, TCAs were thought to exert their analgesic effects mainly through central modulation of both serotonergic and noradrenergic activities.21,22
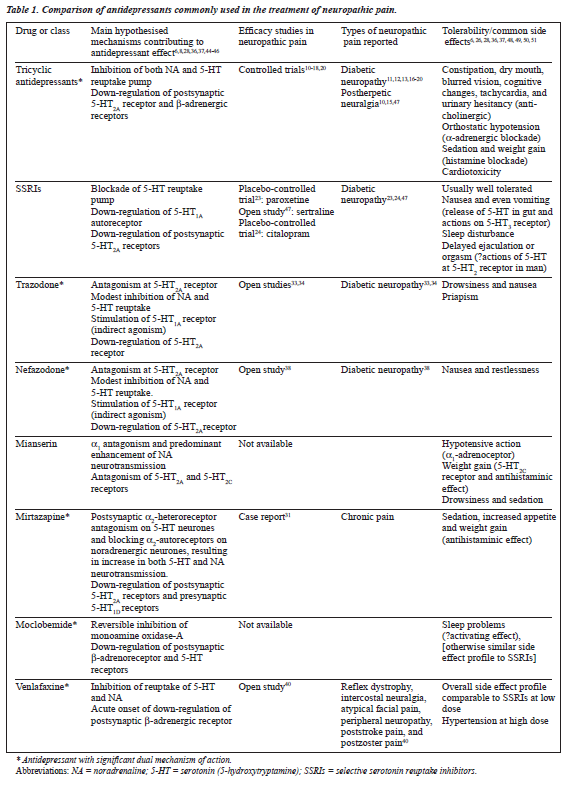
TCAs have diverse actions through their effects on different receptors. The sedative effect of TCAs is some- times useful for sleep disturbance, but the anticholinergic, cardiotoxic, and orthostatic effects may be problematic. In the study by Kvinesdal et al described earlier, 3 of 15 patients dropped out because of compliance problems and intolerable side effects (dizziness and orthostatic hypotension).11 In view of their better safety and tolerability characteristics, the potential usefulness of newer antidepressants for the treat- ment of neuropathic pain is worthy of further exploration. Comparative properties of antidepressants used in the treatment of neuropathic pain are summarised in Table 1.
Use of Newer Antidepressants
Selective serotonin reuptake inhibitors
SSRIs selectively block the reuptake of serotonin in the presypnaptic bulb, and are reported to have a lower incidence of side effects compared with TCAs. One study demonstrated that paroxetine 40 mg/day was effective for painful diabetic neuropathy.23 In a double-blind, placebo-controlled, crossover study, citalopram 40mg/day was shown to provide slight relief of the symptoms of chronic diabetic neuropathy.24
In a trial comparing desipramine with amitriptyline, and fluoxetine with placebo, Max et al found that fluoxetine 40 mg/day did not appear to be effective for pain in diabetic neuropathy, while amitriptyline and desipramine were each effective, to a similar degree.12 The findings supported the hypothesis that the inhibition of the reuptake of nor- adrenaline may account for the analgesic effect of TCAs. The blockade of serotonin reuptake by amitriptyline might augment the analgesic effect.12,25 In similar fashion to claims regarding antidepressant effects, an enhanced analgesic effect of other antidepressants with dual actions was proposed.
Moclobemide
Moclobemide, a reversible inhibitor of monoamine oxidase- A, appears to be a safer option than conventional monoamine oxidase inhibitors (MAOIs) because it is less likely to induce hypertensive crisis and is less toxic in overdose. Moreover, moclobemide is better tolerated than MAOIs, and has an overall side effect profile comparable to fluoxetine.26 However, the efficacy of moclobemide in treating neuro- pathic pain seems to be limited. In an open-label study in patients with chronic neuropathic pain, a discernible anal- gesic effect was noted only in 1 of 7 patients.27
Mianserin
Mianserin is a tetracyclic antidepressant, with a good safety profile in overdose, and low cardiotoxicity. It is antagonistic at a -adrenoceptors and predominantly enhances nora- drenergic transmission only.8,28 The use of mianserin has been limited by its hypotensive action (due to the a -adrenoceptor blockade) and potent antihistaminic effects. There are no reports to support the clinical effectiveness of mianserin for treatment of neuropathic pain. In a double-blind, placebo- controlled study in which 59 subjects with chronic pain were recruited and the daily dosage of mianserin was 60 to 90 mg, Onghena et al showed no significant pain reduction.29
Mirtazapine
Mirtazapine enhances both noradrenergic neurotransmission and serotonergic neurotransmission by antagonising a - autoreceptors on noradrenergic neurones — which increase noradrenaline release, and a -heteroreceptors on serotonin (5-hydroxytryptamine; 5-HT) nerve terminals. The increased levels of noradrenaline act on a -adrenoceptors on the serotonergic cell body to increase serotonergic cell firing. The onset of antidepressant action with mirtazapine was reported to be more rapid than SSRIs,30 a finding thought to result from this unique mechanism of immediate increase in serotonergic firing rate. In contrast to mianserin, mirtaza- pine lacks the a -adrenoceptor antagonism that is associated with postural hypotension. Compared with SSRIs and venla- faxine, nausea and anxiety are less likely with mirtazapine because of its 5-HT receptor blockade. The antagonistic action of the drug at 5-HT receptors reduces the potential for sexual dysfunction as a side effect. 28 In relation to the treatment of neuropathic pain with mirtazapine, only a single case report has been published,31 and further evaluation is required.
Trazodone
Trazodone antagonises the 5-HT postsynaptic receptor and also increases the release of noradrenaline via presynaptic a -receptor blockade. Serotonin reuptake blockade by the drug is weak and probably clinically insignificant. Trazodone has many side effects in common with mianserin. In addition, it has been found to cause priapism in approximately 1 in 6000 male patients.32 Studies using trazodone in neuropathic pain have yielded conflicting results. Khurana33 and Wilson34 reported on the drug’s usefulness in diabetic neuropathy. The pain-relieving effect appeared to be rapid in onset33 and occurred at doses as low as 50 to 100 mg/day.34 On the other hand, in an 8-week, double-blind, placebo-controlled trial of 37 patients, Tammiala-Salonen and Forssell found that trazodone failed to relieve burning mouth pain.35
Nefazodone
Nefazodone exerts a dual mechanism of action in relation to the serotonergic system. It is a selective 5-HT antagonist with strong 5-HT blocking activity, and also inhibits serotonin presynaptic uptake.36 Nefazodone modestly inhibits reuptake of noradrenaline. It is safe in overdose, and does not block anticholinergic receptors. Sleep continuity is selectively improved, an effect possibly related to the 5-HT receptor blockade, and less sexual dysfunction is reported because of the 5-HT receptor inhibition and 5-HT reuptake blockade.28,37 Compared with trazodone, nefazodone is less sedative (because of a lack of antihistaminic activity) and is less likely to cause priapism.36 In a recent open study, Goodnick et al demonstrated that at a mean dose of 340 mg/day, nefazodone was effective in relieving the pain, parasthesis, and numbness associated with diabetic neuropathy.38
Venlafaxine
Venlafaxine is a strong reuptake inhibitor of both serotonin and noradrenaline. At low doses, it is mainly a serotonin reuptake inhibitor, associated with similar side effects as SSRIs. At high doses, it also inhibits noradrenaline reuptake, which may result in increased blood pressure. Venlafaxine was shown to be effective in the treatment of depression and was suggested to have a rapid onset, which might be explained by the acute onset of the down-regulation of b-adrenergic receptors.36 On the other hand, the anti cholinergic, histamin- ergic, and adrenergic activities of venlafaxine are minimal, and thus it is better tolerated than the TCAs. Nausea seems to be a common side effect in the initial treatment phase.39 Blood pressure monitoring is advisable when using venlafaxine, especially in high doses.
There are several reports of the effectiveness of venlafaxine in the treatment of chronic pain. In the open study by Taylor and Rowbotham, involving 12 patients with chronic pain, all 7 with neuropathic pain responded with mild to moderate relief. 40 The dosage ranged from 37.5 mg to 250 mg/day in these patients. In 1999, Davis and Smith reported a patient with painful peripheral neuropathy syndrome who had a dra- matic response to venlafaxine 75 mg/day. 41 That patient was reported to have nearly complete relief of pain within 5 days.
Discussion
Which is the Most Efficacious Antidepressant?
The number needed to treat (NNT) approach42 has been used recently in various reviews comparing the efficacy of dif- ferent pharmacological treatment for pain conditions in controlled studies.5,21,43 The NNT is the number of patients needed to be treated with a certain drug to obtain one patient with a certain level of pain relief. Compared with other approaches such as effect size, NNT is more readily interpretable in clinical settings and more helpful in decision- making. The efficacy of TCAs for the treatment of neuropathic pain is supported by numerous randomised, double- blind, controlled trials in several pain conditions. While the favourable outcome has been defined as a measure equivalent to more than 50% of pain relieved, the NNT was calculated as 2.9 (range, 2.4 to 3.7) [overall] for TCAs, 19 as 2.9 for 5 and as 7.7 for citalopram. 5It was suggested that the NNT for TCA would be even smaller if optimal plasma drug concentrations could be achieved.5 Among the SSRIs, paroxetine seems to be most promising.
Many studies of the newer antidepressants in treatment of neuropathic pain are limited to open-label studies or case reports. Interpretation of these is difficult, and factors such as the dosage and use of concomitant medications are not controlled. Double-blind placebo-controlled clinical trials are needed to evaluate the potential value of these agents in the treatment of neuropathic pain. It also appears premature to conclude that a dual mechanism of action increases anti- depressant efficacy. This situation is not surprising, because the serotonergic and noradrenergic systems interact closely both anatomically and pharmacologically, and attempts to focus on the dichotomy of these 2 systems is likely to be incomplete.
Clinical Implications: Selection of a Suitable Antidepressant
Based on the currently available evidence, TCAs remain the first-line antidepressant treatment for neuropathic pain. However, the side effect profiles of these agents need to be fully explained and monitored because the incidence of side effects is high and may lead to early discontinuation. Also, there is a lack of evidence supporting maintenance use of TCAs in neuropathic pain. Regular evaluation of the effectiveness of TCAs should be done; if TCAs are found to be ineffective or the side effects cannot be tolerated, a trial of one of the newer antidepressants would seem to be worth- while. Meanwhile, a high index of suspicion for detection of depression should be maintained while treating patients with chronic pain. For depressed patients, the assessment of suicide risk should be taken seriously. If the patient has a high suicide risk, the balance of clinical effectiveness against risk of overdose should be carefully evaluated.
The choice among the newer antidepressants depends a lot on their side effect profiles. SSRIs are associated with a higher incidence of nervousness, anxiety, and insomnia when compared with nefazodone or mirtazapine. Drowsiness is more likely to be associated with mirtazapine, postural orthostatic hypotension with nefazodone, and hypertension with venlafaxine. SSRIs and venlafaxine are associated with sexual dysfunction.
Conclusions
Although TCAs are widely used and appear to be the most efficacious agents among the pharmacological treatments of neuropathic pain, unselected long-term use of such agents is not justified. Treatment response, tolerability, and safety should
be considered in clinical settings. A high index of suspicion is required for detection of comorbid depression and suicidal tendency. The effectiveness of the agents prescribed should be reviewed regularly. While the evidence for the efficacy of newer antidepressants is accumulating, paroxetine seems to be the best comparable alternative to TCAs. Other agents, such as trazodone, nefazodone, mirtazapine, and venlafaxine are potentially useful. These agents should therefore be included in comparative trials in the future.
References
1. Serra J. Overview of neuropathic pain syndromes. Acta Neurol Scand Suppl 1999;173:7-11.
2. Hansson P, Lacerenza M, Marchettini P. Aspects of clinical and experimental neuropathic pain: the clinical perspective. In: Hansson PT, Fields HL, Hill RG, Marchettini editors. Neuropathic pain: patho- physiology and treatment. Progress in pain research and Seattle: International Association for the Study of Pain; 2001:1-18.
3. Attal N, Bouhassira D. Mechanisms of pain in peripheral neuropathy. Acta Neurol Scand Suppl 1999;173:12-24.
4. Kumar D, Marshall HJ. Diabetic peripheral neuropathy: amelioration of pain with transcutaneous electrostimulation. Diabetes Care 1997;20:1702-1705.
5. Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuro- pathic pain: an update and effect related to mechanism of drug action. Pain 1999;83:389-400.
6. Nelson JC. Safety and tolerability of the new antidepressants. J Clin Psychiatry 1997;58(Suppl 6):26-31.
7. Kuhn R. The treatment of depressive states with G22355 (imipramine hydrochloride). Am J Psychiatry 1958;115:459-464.
8. Frazer A. Antidepressants. J Clin Psychiatry 1997;58(Suppl 6):9-25.
9. Woodforde JM, Dwyer B, McEwen BW, et al. Treatment of post-herpetic neuralgia. Med J Aust 1965;2:869-872.
10. Watson CP, Evans RJ, Reed K, et al. Amitriptyline versus placebo in postherpetic neuralgia. Neurology 1982;32:671-673.
11. Kvinesdal B, Molin J, Frøland A, et al. Imipramine treatment of painful diabetic neuropathy. JAMA 1984;251:1727-1730.
12. Max MB, Lynch SA, Muir J, et al. Effects of despiramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med 1992;326:1250-1256.
13. Max MB, Culnane M, Schafer et Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37:589-596.
14. Max MD, Schafer SC, Culnane M, et al. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology 1988;38:1427-1432.
15. Kishore-Kumar R, Max MB, Schafer RC, et al. Desipramine relieves post-herpetic neuralgia. Clin Pharmacol Ther 1990;47:305-312.
16. Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of despiramine in painful diabetic neuropathy: a placebo-controlled trial. Pain 1991;45:3-9.
17. Gomez-Perez FJ, Rull JA, Dies H, et al. Nortriptyline and fluphenazine in the symptomatic treatment of diabetic neuropathy: a double-blind cross- over study. Pain 1985;23:395-400.
18. Langohr HD, Stohr M, Petruch F. An open and double-blind cross-over study on the efficacy of clomipramine (Anafranil) in patients with painful mono- and polyneuropathies. Eur Neurol 1982;21:309-317.
19. McQuay HJ, Tramer M, Nye BA, et al. A systemic review of antidepressants in neuropathic pain. Pain 1996;68:217-227.
20. Basbaum AI, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol 1978;4:451-462.
21. Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci 1991;14:219-245.
22. Max MB, Culnane M, Schafer SC, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology 1987;37:589-596.
23. Sindrup SH, Gram LF, Brøsen K, et al. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain 1990;42:135-144.
24. Sindrup SH, Bjerre U, Bejgaard A, et al. The selective serotonin reuptake inhibitor citalopram relieves the symptoms of diabetic neuropathy. Clin Pharmacol Ther 1992;52:547-552.
25. Watson CPN, Chipman M, Reed K, et al. Amitriptyline versus maprotiline in postherpetic neuralgia: a randomised, double-blind, crossover trial. Pain 1992;48:29-36.
26. Williams R, Edwards RA, Newburn GM, et al. A double-blind comparison of moclobemide and fluoxetine in the treatment of depressive disorders. Int Clin Psychopharmacol 1993;7:155-158.
27. Menkes DB, Fawcett JP, Busch AF, et al. Moclobemide in chronic neuro- pathic pain: preliminary case reports. Clin J Pain 1995;11:134-138.
28. Stahl SM. Essential psychopharmacology — neuroscientific basis and practi- cal applications, 2nd ed. New York: Cambridge University Press;
29. Onghena P, De Cuyper H, Van Houdenhove B, et al. Mianserin and chronic pain: a double-blind placebo-controlled process and outcome study. Acta Psychiatr Scand 1993;88:198-204.
30. The role of mirtazapine in the pharmacology of depression [academic highlights]. J Clin Psychiatry 2000;61:609-616.
31. Brannon GE, Stone KD. The use of mirtazapine in a patient with chronic pain. J Pain Symptom Manage 1999;18:382-385.
32. Warner M, Peabody C, Whiteford H, et al. Trazodone and priapism. J Clin Psychiatry 1987;50:256-261.
33. Khurana RC. Treatment of painful diabetic neuropathy with trazodone [letter]. JAMA 1983; 250: 1392.
34. Wilson RC. The use of low-dose trazodone in the treatment of painful diabetic neuropathy. J Am Podiatr Med Assoc 1999;89:468-471.
35. Tammiala-Salonen T, Forssell H. Trazodone in burning mouth pain: a placebo-controlled, double-blind study. J Orofac Pain 1999;13:83-88.
36. Feighner JP. Mechanism of action of antidepressant medications. J Clin Psychiatry 1999;60(Suppl 4):4-11.
37. Goodwin GM. How do antidepressants affect serotonin receptors? The role of serotonin receptors in the therapeutic and side effect profile of the SSRIs. J Clin Psychiatry 1996;57(Suppl 4):9-13.
38. Goodnick PJ, Breakstone K, Kumar A, et al. Nefazodone in diabetic neuropathy: response and biology [letter]. Psychosom Med 2000;62: 599-600.
39. Rudolph RL, Derivan AT. The safety and tolerability of venlafaxine hydrochloride: analysis of the clinical trials database. J Clin Psycho- pharmacol 1996;16(Suppl 2):54S-59S.
40. Taylor K, Rowbotham MC. Venlafaxine hydrochloride and chronic West J Med 1996;165:147-148.
41. Davis JL, Smith RL. Painful peripheral diabetic neuropathy treated with venlafaxine HCL extended release capsules [letter]. Diabetes Care 1999;22:1909-1910.
42. Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452-454.
43. McQuay H, Carroll D, Jadad AR, et al. Anticonvulsant drugs for management of pain: a systemic review. BMJ 1995;311:1047-1052.
44. Samunaris A, Hesselink JK, Pinder R, et al. Development of new antidepressants. J Clin Psychiatry 1997;58(Suppl 6):40-53
45. Song F, Freemantle N, Sheldon TA, et al. Selective serotonin reuptake inhibitors: meta-analysis of efficacy and acceptability. BMJ 1993;306: 683-687.
46. Stahl SM. Is receptor down regulation linked to the mechanism of action of antidepressant drugs? Psychopharmacol Bull 1994;30:39-43.
47. Goodnick PJ, Jimenez I, Kumar A. Sertraline in diabetic neuropathy: preliminary results. Ann Clin Psychiatry 1997;9:255-257.
48. Schweizer E, Feighner J, Mandos L, et al. Comparison of venlafaxine and imipramine in the acute treatment of major depression in J Clin Psychiatry 1994;55:104-108.
49. Cunningham LA, Borison RL, Carman JS, et al. A comparison of venlafaxine, trazodone, and placebo in major depression. J Clin Psycho- pharmacol 1994;14:99-106.
50. Onghena P, Cuyper HD, Houdenhove BV, et al. Mianserin and chronic pain: a double-blind placebo-controlled process and outcome study. Acta Psychiatr Scand 1993;88:198-204.
51. Bazire S. Psychotropic Drug Directory 2000. Dinton: Quay Books;