East Asian Arch Psychiatry 2015;25:73-78
ORIGINAL ARTICLE
Dr Sandeep Grover, MD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Dr Nandita Hazari, MD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Dr Subho Chakrabarti, MD, FRCPsych, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Dr Ajit Avasthi, MD, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India.
Address for correspondence: Dr Sandeep Grover, Department of Psychiatry, Postgraduate Institute of Medical Education and Research, Chandigarh
160012, India.
Tel: (91-172) 2756807; Fax: (91-172) 2744401 / 2745078; Email:drsandeepg2002@yahoo.com
Submitted: 1 September 2014; Accepted: 13 November 2014
Abstract
Objective: To study the prevalence and incidence of seizures in patients prescribed clozapine.
Methods: The treatment records of 222 patients commenced on clozapine were retrospectively reviewed during the period of January 2007 to June 2014 to evaluate the prevalence of seizures before and after starting clozapine.
Results: The majority of patients commenced on clozapine were male (65%), single (65%), and unemployed (57%). The mean (± standard deviation) dose of clozapine was 277.9 ± 102.5 mg/day. A history of seizure was present in 6 patients who were also prescribed antiepileptic medication; of these 6 patients, only 1 case had recurrence of seizure while taking clozapine due to poor compliance with ongoing antiepileptic medication. The incidence rate of new-onset seizure with clozapine was 6% (12/216). Most patients who developed seizures were male, aged between 24 and 41 years, and had a long duration of illness (≥ 10 years). The risk of seizure was associated with the dose of clozapine used: 3% (5/159) with dose up to 300 mg/day, 8% (4/49) with 325 to 500 mg/day, and 38% (3/8) in those receiving > 500 mg/day. More than half of the patients (7/12) who developed seizures while prescribed clozapine were managed with reduction in the dose of clozapine. In one-third of cases (4/12) an antiepileptic medication was added and in 1 case, clozapine was stopped. All patients who continued on clozapine remained seizure-free at follow-up that ranged from 6 months to 4 years.
Conclusion: The incidence of seizures with clozapine was 6% and the risk of seizures increased with higher doses.
Key words: Clozapine; Drug-related side effects and adverse reactions; Seizures
Introduction
Clozapine is an atypical antipsychotic agent most commonly used in the management of treatment-resistant schizophrenia. Its use is indispensable in this subgroup of patients. Nonetheless it is associated with serious side-effects such as blood dyscrasias and seizures that occasionally necessitate withdrawal of treatment.
The incidence rate for clozapine-associated seizures varies from 1.9% to 7.4%, with a cumulative 4-year risk of 10%.1-7 In terms of seizure type, clozapine has been most commonly linked with generalised tonic-clonic seizure (GTCS) although there are also reports of partial, atonic, and myoclonic seizures with clozapine.2,4,6 In some cases the GTCS is reported to be preceded by myoclonic seizures.7 The median time of occurrence of clozapine-induced seizure has been reported to vary from 34 to 75 days5,7 after clozapine initiation, although seizures have occurred as late as 5 years after starting therapy.2
The risk or predisposing factors associated with clozapine-induced seizures include pre-existing seizure disorder, underlying neurological abnormalities, as well as concomitant use of other epileptogenic medications2,8 and selective serotonin reuptake inhibitors (SSRIs) such as sertraline.9 Although inconclusive, higher doses of medi- cations and rapid titration of clozapine have also been linked to clozapine-induced seizures. According to one report, the risk of seizures is 4.4% in patients receiving clozapine at a dose of > 600 mg/day, 2.7% in those receiving 300 to 600 mg/day, and 1% in those receiving < 300 mg/day.2 Nonetheless certain reports suggest that seizures may not be related to dose of medication but to plasma level.5,10,11 A recent study showed that certain patients may have a genetic vulnerability to develop seizure with clozapine. Clozapine-associated seizure activity in this study was linked to CYP1A2*1F/*IF.12 Another study reported a higher incidence of seizures in patients with schizophrenia
In terms of management of clozapine-associated seizures, possible strategies include reduction of clozapine dose and addition of antiepileptic drugs (AEDs).2 Among the various AEDs, valproate is the most commonly used for management of clozapine-induced seizures due to its additional effect as a mood stabiliser,14 minimal effect on metabolism of clozapine,15,16 and reduction in generalised polyspike and wave electroencephalogram (EEG) pattern in patients on clozapine.5,17 Recent guidelines also suggest prophylactic use of valproate in patients prescribed clozapine of > 600 mg/day.18 Evidence also supports the use of lamotrigine,19 gabapentin,20 and topiramate21 as AEDs in patients with clozapine-related seizures.
Pharmacogenomic studies suggest that there may be pharmacokinetic and pharmacodynamic differences in the metabolism and side-effect profile in various ethnic groups.22 Patients of Indian origin may require lower doses of antipsychotics to respond and develop side-effects at lower doses of antipsychotics compared with those used in subjects from the West.23,24 It is thus important to understand the incidence of clozapine-induced seizures and its relationship with clozapine dose in Indian patients.
Only few studies from India have examined clozapine- associated seizures. One study evaluated EEG changes in 87 patients receiving clozapine and that epileptiform activity was reported in 41.4%.17 In a case series of 12 patients with treatment-resistant schizophrenia (TRS) who were receiving clozapine at a dose of > 500 mg/day, drug-induced seizure was noted in 1 patient and EEG abnormalities in 2.25 In a previous study from our centre, which included 51 patients, seizure was observed in 4 patients.26 In view of the limited data from India, this study evaluated the incidence of seizures in patients receiving clozapine and evaluated the prevalence of seizures prior to starting clozapine.
Methods
The study was carried out in a multi-speciality tertiary care hospital in North India where most patients with schizophrenia were treated on an outpatient basis. In our unit, clozapine was usually used in patients with TRS or in those who were unable to tolerate other antipsychotics. In most cases clozapine was commenced in the inpatient setting. Before starting clozapine, the patient had undergone detailed evaluation for treatment resistance, as well as co- morbid physical and psychiatric disorders. Evaluation included routine haematology, liver function tests, fasting blood sugar, lipid profile, electrocardiogram, and EEG. Clozapine was usually started at a dose of 12.5 to 25 mg/ day, gradually increased while monitoring side-effects and response to treatment. Most patients usually stabilised at a dose of 150 to 350 mg/day, with occasional patients requiring a higher dose. Once stabilised, the patient was followed up regularly with haematological monitoring. In the event of serious side-effects such as seizure, cardiac arrhythmias, or blood dyscrasias, relevant investigations were initiated to exclude any other aetiology.
All clinical details of the patient during inpatient and outpatient care were entered into the same notes. Data pertaining to the diagnosis and treatment given while the patient was hospitalised were also entered into a computer- based registry.
This study followed a retrospective study design. The inpatient registry for the period of January 2007 to June 2014 was used to identify patients who were commenced on clozapine or who continued on clozapine during the inpatient stay. In addition, individual consultants were contacted to collect information about any patient who commenced clozapine as an outpatient. On the basis of available information, the treatment records of 222 patients were reviewed and information retrieved for socio- demographic profile, clinical details, co-morbid epilepsy, and development of seizures after starting clozapine.
Based on clinical data, patients receiving clozapine were evaluated for a history of epilepsy / seizure and new onset of seizure after starting clozapine. For this study, seizures were considered to be associated with the use of clozapine when the patient had a normal EEG prior to treatment and no other underlying cause for development of seizures. The study was approved by the Institute Ethics Review Board.
Results
Treatment records of 222 patients receiving clozapine were reviewed. Follow-up data for at least 3 months after starting clozapine were available for all patients, with the longest follow-up data available for 18 years. Follow-up information for more than 1 year was available in 84% (n = 186) of cases.
The mean (± standard deviation) age of the study sample was 33.5 ± 11.3 years (range, 15-67 years). About two-thirds (65%; n = 145) of the study sample was male. Most patients were single (65%; n = 145), Hindu by religion (74%; n = 164), and more than half were unemployed (57%; n = 126). About three-quarters were from nuclear families (73%; n = 162), from an urban background (74%; n = 165), and had a family income of > 6,000 rupees (about US$94) per month (77%; n = 171).
The mean age of onset of psychiatric illness was 23.03 ± 9.42 years and the mean duration of illness prior to starting clozapine was 121.5 ± 89.9 months. Almost all the study subjects suffered from schizophrenia, with paranoid schizophrenia (n = 107; 48%) and undifferentiated schizophrenia (n = 75; 34%) forming the major proportion of the study sample. Few patients were diagnosed with bipolar disorder (n = 5), persistent delusional disorder (n = 3), and recurrent depressive disorder with tardive dyskinesias (n = 3).
The mean dose of clozapine was 277.9 ± 102.5 mg/day (range, 75-700 mg/day). Almost three-quarters (74%; n = 165) of patients were prescribed clozapine at a dose of up to 300 mg/day, 22% (n = 49) were prescribed 325 to 500 mg/day, and 4% (n = 8) received > 500 mg/day.
Of these 222 patients, 6 patients had a history of seizures prior to starting clozapine and were prescribed AEDs alongside: valproate in 4 patients, and lamotrigine and topiramate in 1 case each. Of these 6 patients, 1 case had recurrence of GTCS after starting clozapine at the dose of 200 mg/day for 1 month, alongside poor compliance with topiramate at the time. On further follow-up for the next year, the patient remained seizure-free while receiving clozapine and topiramate. In the remaining 5 patients prescribed clozapine while maintained on AEDs, no recurrence of seizure was noted for the next 1 to 4 years of follow-up.
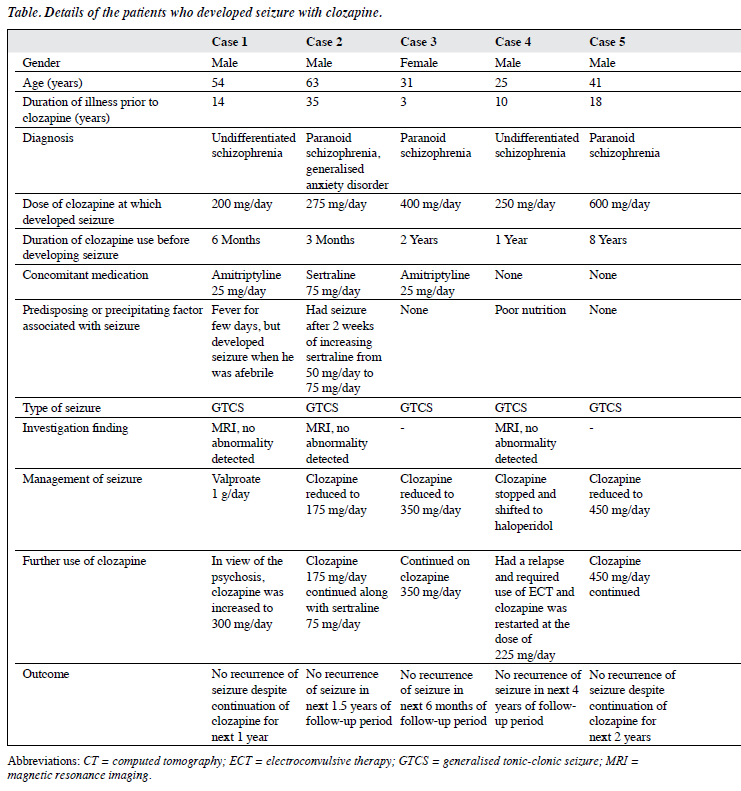
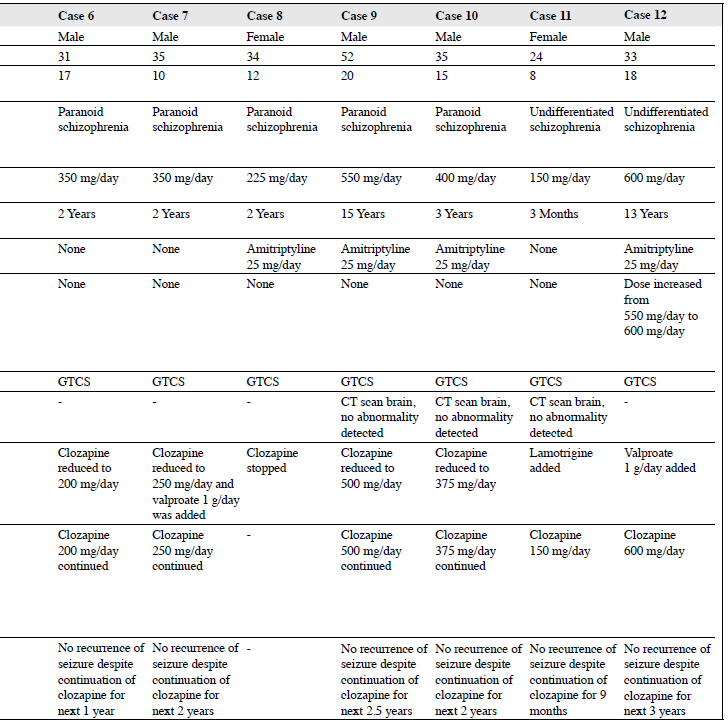
Of the remaining 216 patients, 12 (6%) developed seizure while on clozapine, details of whom are shown in the Table. All these patients were prescribed clozapine because of TRS and most (75%) were male, aged between 24 and 41 years (75%), and had had schizophrenia for ≥ 10 years (83%) of which paranoid schizophrenia (67%) was the most common diagnosis. The dose of clozapine at which patients developed seizure varied from 150 to 600 mg/day; and the majority (n = 11) developed seizures after starting clozapine after 6 months of therapy. Of these 12 patients, 6 were receiving concomitant amitriptyline at a dose of 25 mg/day that was usually prescribed in our unit for the management of clozapine-induced hypersalivation. Nonetheless all these patients had been taking amitriptyline long before they developed seizure. One patient was receiving concomitant sertraline along with clozapine at the time of development of seizures. In terms of predisposing factors or factors that could contribute to development of seizure, 1 patient had a history of fever a few days before developing seizure. Nonetheless, neurological examination, neuroimaging and other blood investigations revealed no electrolyte imbalance. In another case, seizure occurred 1 month after the dose of clozapine was increased from 550 mg/day to 600 mg/day. In the third case, seizure was preceded by an increase in the dose of sertraline from 50 mg/day to 75 mg/day and yet another case had poor nutritional status prior to developing seizure. All patients had GTCS while receiving clozapine and in those who underwent further evaluation, no abnormality was noted on neuroimaging. In terms of management of clozapine-associated seizures, in most cases (7/12) the dose of clozapine was reduced by 25 to 150 mg/day, following which there was no recurrence of seizures. Antiepileptic drug in the form of valproate was added in 3 cases, lamotrigine was added in 1 case, and in 1 case clozapine was stopped. Electroconvulsive therapy was subsequently administered to a patient without any complications and clozapine was restarted. In 1 case the dose of clozapine was increased further under the cover of valproate to manage symptoms of psychosis. All these patients had no recurrence of seizure over the follow- up period of 6 months to 4 years despite continuation of clozapine after the seizure episode.
There was no significant difference in the mean dose of clozapine between those who had seizures (329.2 ± 110.2 mg/day) and those who did not have seizures with clozapine (276.3 ± 102.4 mg/day; t = 1.73, p = 0.09). Nonetheless evaluation of the risk of seizure with respect to dose of clozapine revealed that a higher incidence was evident with higher doses. The risk of seizure was 3% (5/159) with clozapine dose up to 300 mg/day, 8% (4/49) in those receiving clozapine in the dose range of 325 to 500 mg/day, and 38% (3/8) in those receiving clozapine > 500 mg/day.
Discussion
This study evaluated the risk of developing seizures with clozapine. The findings suggest that 6% of patients prescribed clozapine developed seizures. This finding falls in the reported range of 1.9% to 7.4%.1-7
Some of the previous reports suggest the association of seizures with dose of clozapine. One study noted the risk of seizure to be 1% in those receiving clozapine of < 300 mg/day, 2.7% in those receiving 300 to 600 mg/day, and 4.4% in those receiving clozapine of > 600 mg/day.2 Our study findings provide further evidence of dose-related seizure activity with clozapine. In our study 3% of patients prescribed clozapine up to 300 mg/day had seizures and this risk increased to 8% in those receiving a dose of 325 to 500 mg/day. The risk of seizures was maximum, i.e. 38%, in those receiving clozapine of > 500 mg/day. When we compare our findings with the previous study,2 it can be concluded that at equivalent doses, the risk of seizure is higher in Indian patients compared with those from the West. It is likely that this difference is due to genetic differences in various ethnic groups. A previous study from India also reported seizure activity in 1 out of 12 patients and abnormal EEG in another 2 patients who were prescribed > 500 mg/day clozapine.25
Accordingly we suggest that, whenever possible, when treating patients of Indian origin clinicians should prescribe a relatively lower dose of clozapine compared with that in patients from western countries. When a clinician prescribes clozapine at a dose of > 300 mg/day, it is important to inform the patient and caregivers about the relatively higher risk of seizures and, if required, monitor the patient with EEGs. Certain western guidelines27 suggest prophylactic use of valproate in patients prescribed clozapine of ≥ 600 mg/day. We recommend that in Indian patients this threshold be kept lower, maybe at 400 mg/day.
Evidence suggests that in most patients clozapine- associated seizures manifest as GTCS,2,4,6 as was the case in all our patients. Nonetheless in contrast to previous studies,5,7 most of our patients developed seizures while on a stable dose of clozapine for at least 6 months, with 1 patient developing seizure after 15 years of clozapine therapy. In terms of risk factors, half of our patients were concomitant amitriptyline. It is unlikely that this could have contributed to seizure since, in most cases, amitriptyline therapy was of almost the same duration as that of clozapine. Nonetheless in 1 case seizure was precipitated by starting concomitant sertraline. There are previous reports that also suggest a similar association of seizure and sertraline in patients receiving clozapine.9 A most likely reason for this is an increase in the serum level of clozapine due to interaction between clozapine and sertraline. Accordingly, a cautious approach must be followed when combining SSRIs with clozapine and patients must be closely monitored for seizure activity.
In terms of management of clozapine-associated seizures, reduction in dose of clozapine was sufficient in most instances to prevent recurrence of seizure and only 4 patients were treated with AEDs (3 with valproate, 1 with lamotrigine). This finding suggests that whenever a patient develops seizure with clozapine, dose reduction should be the first strategy to prevent further seizures. If this fails or if the patient requires higher doses of clozapine for management of their primary illness, then valproate may be considered.
The findings must be interpreted in light of the study limitations. We followed a retrospective study design and information about seizures was obtained from the treatment record. It is quite possible that this could have led to some underestimation of seizures associated with clozapine. We also did not evaluate the relationship of seizure activity with clozapine serum levels. This study was limited to one treatment centre. We did not evaluate the relationship of seizures with other side-effects of clozapine and the level of treatment response. This study also did not evaluate the genetic factors that may underlie development of seizures. Future studies must attempt to overcome these limitations. There is a need for pharmacogenomic studies to understand the higher risk of seizures with clozapine in Indian patients.
To conclude, the findings of the present study suggest that 6% of patients receiving clozapine developed seizures. This risk increased to about 8% in those receiving clozapine in the dose range of 325 to 500 mg/day and 38% in those receiving clozapine of > 500 mg/day. In most cases of clozapine-associated seizures, dose reduction was sufficient to prevent recurrence of seizures.
References
- Lindström LH. The effect of long-term treatment with clozapine in schizophrenia: a retrospective study in 96 patients treated with clozapine for up to 13 years. Acta Psychiatr Scand 1988;77:524-9.
- Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology 1991;41:369-71.
- Liukkonen J, Koponen HJ, Nousiainen U. Clinical picture and long- term course of epileptic seizures that occur during clozapine treatment. Psychiatry Res 1992;44:107-12.
- Berman I, Zalma A, DuRand CJ, Green AI. Clozapine-induced myoclonic jerks and drop attacks. J Clin Psychiatry 1992;53:329-30.
- Pacia SV, Devinsky O. Clozapine-related seizures: experience with 5629 patients. Neurology 1994;44:2247-9.
- Wilson WH, Claussen AM. Seizures associated with clozapine treatment in a state hospital. J Clin Psychiatry 1994;55:184-8.
- Sajatovic M, Meltzer HY. Clozapine-induced myoclonus and generalized seizures. Biol Psychiatry 1996;39:367-70.
- Toth P, Frankenburg FR. Clozapine and seizures: a review. Can J Psychiatry 1994;39:236-8.
- Phutane VH, Kumar CN, Thirthalli J, Mahato SK, Sinha S. Partial seizures with secondary generalization while on treatment with clozapine and sertraline: a case report. Prim Care Companion J Clin Psychiatry 2009;11:127-8.
- Greenwood-Smith C, Lubman DI, Castle DJ. Serum clozapine levels: a review of their clinical utility. J Psychopharmacol 2003;17:234-8.
- Freudenreich O, Weiner RD, McEvoy JP. Clozapine-induced electroencephalogram changes as a function of clozapine serum levels. Biol Psychiatry 1997;42:132-7.
- Kohlrausch FB, Severino-Gama C, Lobato MI, Belmonte-de-Abreu P, Carracedo A, Hutz MH. The CYP1A2-163C>A polymorphism is associated with clozapine-induced generalized tonic-clonic seizures in Brazilian schizophrenia patients. Psychiatry Res 2013;209:242-5.
- Caluseriu O, Tayyeb T, Chow E, Bassett AS. Clozapine-associated seizures in a 22Q deletion syndrome subtype of schizophrenia. Schizophrenia Res 2003;60:79.
- Foster R, Olajide D. A case of clozapine-induced tonic-clonic seizures managed with valproate: implications for clinical care. J Psychopharmacol 2005;19:93-6.
- Centorrino F, Baldessarini RJ, Kando J, Frankenburg FR, Volpicelli SA, Puopolo PR, et al. Serum concentrations of clozapine and its major metabolites: effects of cotreatment with fluoxetine or valproate. Am J Psychiatry 1994;151:123-5.
- Varma S, Bishara D, Besag FM, Taylor D. Clozapine-related EEG changes and seizures: dose and plasma-level relationships. Ther Adv Psychopharmacol 2011;1:47-66.
- Goyal N, Praharaj SK, Desarkar P, Nizamie H. Electroencephalographic abnormalities in clozapine-treated patients: a cross-sectional study. Psychiatry Investig 2011;8:372-6.
- Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines. 10th ed. London: Informa Healthcare; 2009.
- Muzyk A, Gala G, Kahn DA. Use of lamotrigine in a patient with a clozapine-related seizure. J Psychiatr Pract 2010;16:125-8.
- Landry P. Gabapentin for clozapine-related seizures. Am J Psychiatry 2001;158:1930-1.
- Navarro V, Pons A, Romero A, Bernardo M. Topiramate for clozapine- induced seizures. Am J Psychiatry 2001;158:968-9.
- Silva H. Ethnopsychopharmacology and pharmacogenomics. Adv Psychosom Med 2013;33:88-96.
- Srivastava AK, Gupta S, Srinivasan N. Risperidone: dosing pattern and efficacy in clinical practice — a post-marketing survey. Indian J Psychiatry 2001;43:147-51.
- Avasthi A, Aggarwal M, Grover S, Khan MK. Research on antipsychotics in India. Indian J Psychiatry 2010;52(Suppl 1):S317- S340.
- Mendhekar DN. High dose of clozapine in resistant schizophrenia. Indian J Psychiatry 2001;43:103-4.
- Dutt A, Grover S, Chakrabarti S, Kulhara P, Avasthi A, Basu D, et al. Effectiveness of clozapine: a study from North India. Asian J Psychiatr 2010;3:16-9.
- Taylor D, Paton C, Kapur S. The Maudsley prescribing guidelines in psychiatry. 12th ed. Wiley Blackwell; 2015.