East Asian Arch Psychiatry 2016;26:148-53
ORIGINAL ARTICLE
Dr Preeti Jacob, Department of Child and Adolescent Psychiatry, National Institute of Mental Health And Neuro Sciences, Bangalore, India.
Prof. Shoba Srinath, Department of Child and Adolescent Psychiatry, National Institute of Mental Health And Neuro Sciences, Bangalore, India.
Prof. Satish Girimaji, Department of Child and Adolescent Psychiatry, National Institute of Mental Health And Neuro Sciences, Bangalore, India.
Prof. Shekhar Seshadri, Department of Child and Adolescent Psychiatry, National Institute of Mental Health And Neuro Sciences, Bangalore, India.
Prof. John Vijay Sagar, Department of Child and Adolescent Psychiatry, National Institute of Mental Health And Neuro Sciences, Bangalore, India.
Address for correspondence: Dr Preeti Jacob, Department of Child and Adolescent Psychiatry, National Institute of Mental Health And Neuro Sciences, Bangalore, India.
Tel: (91) 0802 699 5846; Email: preetijacob@gmail.com
Submitted: 19 August 2015; Accepted: 4 January 2016
Abstract
Objective: To assess the prevalence of neurodevelopmental and psychiatric co-morbidities in children and adolescents diagnosed with attention-deficit hyperactivity disorder at a tertiary care child and adolescent psychiatry centre.
Methods: A total of 63 children and adolescents who were diagnosed with attention-deficit hyperactivity disorder and fulfilled the inclusion criteria were comprehensively assessed for neurodevelopmental and psychiatric co-morbidities. The tools used included the Mini-International Neuropsychiatric Interview for Children and Adolescents, Attention Deficit Hyperactivity Disorder Rating Scale IV (ADHD-RS), Children’s Global Assessment Scale, Clinical Global Impression Scale, Vineland Social Maturity Scale, and Childhood Autism Rating Scale.
Results: All except 1 subject had neurodevelopmental and / or psychiatric disorder co-morbid with attention-deficit hyperactivity disorder; 66.7% had both neurodevelopmental and psychiatric disorders. Specific learning disability was the most common co-existing neurodevelopmental disorder and oppositional defiant disorder was the most common psychiatric co-morbidity. The mean baseline ADHD-RS scores were significantly higher in the group with psychiatric co-morbidities, especially in the group with oppositional defiant disorder.
Conclusion: Co-morbidity is present at a very high frequency in clinic-referred children diagnosed with attention-deficit hyperactivity disorder. Psychiatric co-morbidity, specifically oppositional defiant disorder, has an impact on the severity of attention-deficit hyperactivity disorder. Co-morbidity needs to be explicitly looked for during evaluation and managed appropriately.
Key words: Adolescent; Attention deficit disorder with hyperactivity; Child; Comorbidity
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common but complex neurodevelopmental disorder with onset in childhood or adolescence.1,2 Co-morbidity with psychiatric and other neurodevelopmental disorders seems to be a distinct clinical characteristic, although the reasons and mechanisms for the same have not been fully elucidated.3,4
Why is co-morbidity in ADHD so important? Psychiatric and other neurodevelopmental co-morbidities seen with ADHD have been shown to have assessment, diagnostic, management-related, and prognostic implications.5,6
Barkley7 even argues for ADHD with co-morbidities to be thought of as a discrete clinical subtype with its own course and outcome profile, separate from pure ADHD. Although community-based studies may be ideal to identify the prevalence of co-morbidities,8 given the limitations in terms of trained manpower, funding and the magnitude and diversity of the Indian population, good clinic-based naturalistic, observational studies are a viable alternative and an adjunct to community-based studies. There are very few prospective Indian studies of co-morbidity in children with ADHD.9-11 This study was undertaken to assess the prevalence of co-morbidity in children diagnosed with ADHD at a tertiary care child and adolescent psychiatry centre.
Methods
Sample and Setting
This was an observational, prospective study drawn from the clinical sample attending the outpatient services of the Department of Child and Adolescent Psychiatry at the National Institute of Mental Health And Neuro Sciences (NIMHANS), Bangalore, India. The methodology and results presented and discussed in this paper are part of a larger follow-up study. Children and adolescents between 4 and 16 years, who were newly diagnosed by consultant psychiatrists in the department to have ADHD as per the DSM-IV criteria and who were drug-naïve, were included in the study. Data were collected over a period from 1 December 2011 to 30 November 2012. In order to maintain the reliability of the observations, we attempted to include only those families who had a parent / guardian constantly staying with the child. Children who had moderate, severe or profound intellectual disability, any progressive neurological disorder and / or sensory impairment were excluded from the study. This was done as there was considerable diagnostic masking and overshadowing in this population thus it might be difficult to diagnose ADHD and other psychiatric co-morbidities reliably with the tools used in the study.
All tests were administered by the first author to the same parent / guardian throughout the study period. The socio-demographic and clinical profiles were recorded as per a pre-specified format followed by Department of Child and Adolescent Psychiatry, NIMHANS. This included, in addition to history of present illness, physical and psychiatric examinations, a detailed birth history (pre- / peri- / post-natal history), developmental milestones and current developmental level (motor, social, language and adaptive), temperamental characteristics as per Chess and Thomas12 classification, family history of psychiatric illness and / or substance use, parenting style, psychosocial history (marital discord, domestic violence, disciplining practices, family environment), schooling history including academic problems, if any, and attendance at school. These details were obtained from the primary caregiver.
The school teacher was also interviewed by telephone after obtaining the parent and the child’s consent with regard to the child’s problems in school if any, their academic performance in all subjects, and their capacity for interpersonal relationships both with authority figures and peers. The teacher also rated the Attention Deficit Hyperactivity Disorder Rating Scale IV (ADHD-RS) [School version].13 This was administered to the teacher by telephone by the first author.
All children and adolescents included in the study with a history of speech and language problems were evaluated independently by the speech and language pathologist at the Department of Speech Pathology and Audiology, NIMHANS.
The Mini-International Neuropsychiatric Interview for Children and Adolescents (MINI-KID) is a structured diagnostic interview schedule with good reliability and validity.14 It was used to confirm the diagnosis of ADHD and to diagnose psychiatric co-morbidities. This was administered by the first author to the child and parent / guardian during assessment.
The ADHD-RS (Home and School Versions) was used to assess the severity of ADHD symptoms. This scale has good internal consistency and test-retest reliability.13
The Clinical Global Impression Scale (CGI)15 is a clinician- rated instrument and was used to provide a simple and clinically useful measure of change.
The Children’s Global Assessment Scale (CGAS)16 is another clinician-rated instrument with good psychometric properties used to provide a global measure of functioning.
The Vineland Social Maturity Scale (VSMS)17 validated for Indian children was used as a measure of adaptive functioning and social maturity. The VSMS provides an estimate of social age and a social quotient (SQ), and has shown a strong correlation with intelligence.18 In this study SQ was used by analogy to the measure of intelligence.
The Childhood Autism Rating scale (CARS)19 was used to assess the severity of autism in children diagnosed to have pervasive developmental disorder as per the DSM-IV criteria.
The NIMHANS Index was developed at NIMHANS to diagnose children with specific learning disabilities.20 This was administered by clinical psychologists attached to the Department of Child and Adolescent Psychiatry after a clinical diagnosis of specific learning disability according to the ICD-10 criteria made by the treating clinician after the age of 8 years.
Procedure
Written informed consent was obtained from all the parents / guardians and assent where possible was obtained from the children prior to their inclusion in the study. The Institute Ethics Committee approved the study. A detailed history was obtained by the junior resident and discussed with the consultant psychiatrist. After a diagnosis of ADHD was made by the consultant psychiatrist, and confirmation obtained that the child fulfilled the inclusion criteria, they were recruited to the study by the first author. The first author then administered the VSMS to all children at baseline. Those with a SQ of < 50 were excluded from the study for the reasons explained above. Socio-demographic and clinical profiles were obtained through a detailed history from the child and the primary caregiver. The teacher was also interviewed by telephone. The MINI-KID, ADHD-RS, CGI, and CGAS were administered by the first author. After diagnosis, the children were managed as per the usual treatment practices of the clinic. Routine treatment was both pharmacological (if deemed necessary by the treating clinician) and psychosocial. Data were tabulated and analysed using the Statistical Package for the Social Sciences version 21.0 (SPSS Inc., Chicago [IL], US).
Results
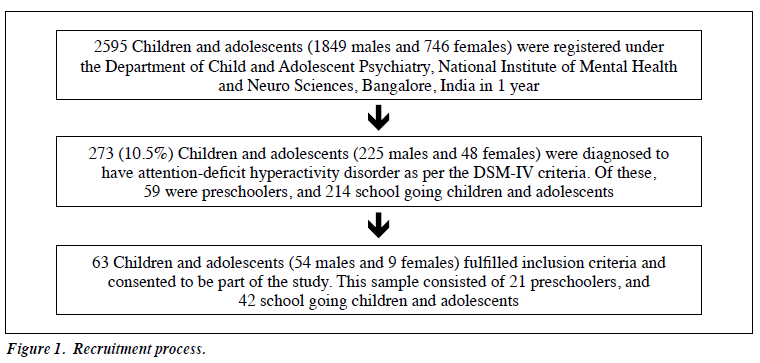
Among 273 subjects diagnosed with ADHD, 63 (23.1%) were included in the study. The study sample comprised 54 males and 9 females. Among them, 21 were aged between 4 and 6 years, 33 between 6.1 and 11 years, and 9 between 11.1 and 16 years. The clinical prevalence of ADHD was 10.5% (Fig 1), with a male-to-female ratio of 4.7:1. The mean (± standard deviation) age of the sample was 7.79 ± 2.83 years. All children in this sample had symptoms of ADHD prior to the age of 4 years, with a mean age at presentation of 2.98 ± 0.53 years. In all, 92.1% were diagnosed with combined, 4.8% with inattentive, and 3.2% with hyperactive-impulsive types of ADHD.
The mean baseline ADHD-RS score was 38.17 ± 7.52 and the mean CGAS score was 48.28 ± 8.13. The CGI severity scale at baseline showed that 63.5% of subjects were moderately ill, 22.2% mildly ill, 11.1% markedly ill, and 1.6% severely / borderline mentally ill.
Birth and Developmental History
A significant birth history was evident in 46% of children; 20.6% had low birth weight (< 2.5 kg). Prenatal factors most commonly cited by parents were anaemia, pregnancy- induced hypertension, hypothyroidism, and antepartum haemorrhage. Perinatal and postnatal factors most commonly cited were fetal distress and delayed birth cry, neonatal jaundice, and need for neonatal intensive care. Developmental delay had been present in 68.3%, of whom 25.4% had a history of global developmental delay and 42.9% had a history of speech delay. All children who were diagnosed with speech delay were referred to the Department of Speech Pathology and Audiology, NIMHANS and received a diagnosis of delayed speech and language.
Medical Co-morbidity
Medical co-morbidity was present in 22.2% of subjects, in whom 12 children were diagnosed with seizure disorder (10 with seizures only, 1 with cerebral palsy and 1 with hypothyroidism). The seizure disorder was well controlled with the last seizure in all children being more than 1 year ago. One child had bronchial asthma and another had a history of glomerulonephritis.
Neurodevelopmental Co-morbidity
A co-morbid neurodevelopmental disorder was diagnosed in 82.5% of study subjects and included intellectual disability, specific learning disability, and autism spectrum disorder / pervasive developmental disorder (Fig 2). In addition, 77.8% (n = 49) had at least 1 and 4.8% (n = 3) had 2 co-morbid neurodevelopmental disorders. Enuresis was present in 57.1% (n = 36).
Mild intellectual disability was evident in 23.8% (n = 15) of children; 41.3% (n = 26) had a SQ between 70 and 90 and were considered slow learners, and 34.9% (n = 22) had a SQ of > 90 and were considered to be of average intelligence.
A total of 21 children were diagnosed by a clinical psychologist to have a specific learning disability according to the NIMHANS Index of Specific Learning Disabilities.20
The 12 children who were under 8 years of age, and who had a history of academic difficulties along with speech delay were considered to be at risk for specific learning disability. Also, 7.9% (n = 5) of children were diagnosed to have pervasive developmental disorder. The CARS was used to assess the severity of autism. Four children were rated to have mild-to-moderate autism (30-36.5) and 1 was rated as having severe autism. Of the 5 children with pervasive developmental disorder, 3 had mild intellectual disability (SQ < 70) and 2 had a SQ of 70 to 90 and were considered slow learners.
Psychiatric Co-morbidity
Psychiatric co-morbidity was diagnosed in 82.5% of the study sample, with a mean of 1.9 ± 0.77 psychiatric co- morbidities. Details of their psychiatric co-morbidities are depicted in Table 1.
There was a considerable degree of overlap between the psychiatric co-morbidities seen with ADHD. All the study subjects with conduct disorder (CD) also had co- morbid oppositional defiant disorder (ODD). Both ODD and CD are considered disruptive behaviour disorders (DBD). In all, 34.9% (n = 22) were diagnosed with both DBD and an anxiety disorder with ADHD; 38.1% (n = 24) were diagnosed to have co-morbid DBD alone with ADHD; and 9.5% (n = 6) were diagnosed to have an anxiety disorder alone with ADHD.
Table 2 shows that the baseline mean ADHD-RS (Home version) scores were significantly higher in the group with psychiatric co-morbidities (p = 0.001) and those with co-morbid ODD (p = 0.001). Among the study sample (n = 63), the mean baseline ADHD-RS scores were highest in the group with ODD, followed by those with anxiety and ODD, then those with anxiety, and least in the group with neither of these co-morbidities with ADHD. The mean baseline ADHD-RS scores were not significantly higher in the group with co-morbid neurodevelopmental disorders, specific learning disabilities, pervasive developmental disorders and anxiety disorders, or the group without these co-morbidities. There was a significant negative correlation between the baseline CGAS score and baseline ADHD-RS score (Pearson’s correlation coefficient r = –0.311, p = 0.013). The higher the CGAS score the lower the total ADHD-RS score at baseline.
In all, 85.7% of subjects had academic problems, of whom 80.9% had another neurodevelopmental disorder as discussed above and 4.8% had academic difficulties but no co-morbid neurodevelopmental disorder.
Overall, specific learning disability was the most common co-existing neurodevelopmental disorder and oppositional defiant disorder was the most common psychiatric co-morbidity.
Discussion
The child and adolescent psychiatry services at NIMHANS are unique in that we see an equal measure of patients with neurodevelopmental and psychiatric disorders. While co-morbidities in clinic settings are more frequent,8 it is now understood that co-morbidities in ADHD occur quite commonly in all settings and in diverse populations.21,22
Although it has been depicted pictorially (Fig 2), it needs to be reiterated that there was only 1 subject in the entire sample who had no neurodevelopmental or psychiatric co- morbidity with ADHD. As suggested aptly by Angold et al,23 co-morbidity is truly the rule rather than the exception, especially in this study.
With respect to neurodevelopmental disorders, although they did not seem to worsen the severity of ADHD in this study (Table 2), they were seen in a significant proportion of the study population. Most previous studies in children with ADHD excluded neurodevelopmental disorders, especially intellectual disability and pervasive developmental disorders, and thus there is a paucity of literature in this area.24,25 Attention-deficit hyperactivity disorder itself has been reconceptualised as a neurodevelopmental disorder in DSM-5.2 This is a welcome step and although it is seen only in a small measure in this study, the degree of overlap between the different neurodevelopmental disorders is vast. The reconceptualisation of ADHD should hopefully revitalise and fuel research in this area and be able to inform real- world practice.26
In this study, the rates of psychiatric co-morbidity were similar to that in other clinic-based studies.21,27 Oppositional defiant disorder was the most frequent psychiatric co- morbidity and did impact the severity of ADHD symptoms.
This finding is similar to that of other studies.9-11,27 Anxiety disorders are often underdiagnosed in children with ADHD as they are at divergent ends of the behaviour spectrum being an internalising disorder. Nonetheless, they do occur quite frequently with ADHD and need to be specifically looked for as there are both management and prognostic implications.28,29
Another significant finding in this study was the large number of children with speech delay and academic problems. This has also been documented in the literature.30
It has also been shown that children with specific learning disabilities with ADHD may appear more inattentive and therefore the clinician needs to be careful while assessing and managing children with this dual co-morbidity.30
From the above study it is evident that co-morbidities occur with alarming frequency in clinic-referred children and thus a careful and systematic assessment is mandatory both for the ADHD and for the other co-existing disorders. This study adds to the body of literature which states that premature closure after making a diagnosis of ADHD without identifying and addressing the co-morbidities will have a significant negative impact on the long-term outcome.5,31 Often children are prescribed medication for the ADHD when most of the academic / school-related problem behaviours stem from the specific learning disability that has been missed.
The strength of the study was that all evaluations were done using a structured interview format by a trained child psychiatrist. The study was conducted in a clinic population and thus has implications for routine clinical practice. One of the limitations of this study was that the assessments were not conducted by blind raters and may have resulted in observer bias. Another limitation was that there were very few adolescents (n = 9) in the sample studied, thus co-morbidities like mood disorders and substance use disorders were not seen. Only 23.1% (63 / 273) of children diagnosed with ADHD from the clinic could be included in the study for a variety of reasons. This imposes limits on the generalisability of the results. It is imperative that a thorough evaluation for co-morbid neurodevelopmental and psychiatric disorders be carried out for comprehensive management of ADHD.
Conclusion
Co-morbidity seems to be the rule rather than the exception in clinic-referred children diagnosed with ADHD. Clinicians who diagnose children with ADHD must screen them for other neurodevelopmental and psychiatric co-morbidities. Consequently the intervention package designed for these children must be all-inclusive and should address both the primary disorder as well as the concurrent co-morbidity.
Declaration
The authors declared no conflicts of interest or disclosures in this study.
References
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit / hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA 1998;279:1100-7.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition: DSM-5. Arlington, VA: American Psychiatric Publishing; 2013.
- Spencer TJ, Biederman J, Mick E. Attention-deficit / hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol 2007;32:631-42.
- Cooper M, Thapar A. Shared biological risks that influence brain and behaviour. Arch Dis Childhood 2012;97:1011-2.
- Jensen PS, Martin D, Cantwell DP. Comorbidity in ADHD: implications for research, practice, and DSM-V. J Am Acad Child Adolesc Psychiatry 1997;36:1065-79.
- Larson K, Russ SA, Kahn R, Halfon N. Patterns of comorbidity, functioning and services uses for US children with ADHD, 2007. Pediatrics 2011;127:462-70.
- Barkley RA. Comorbid disorders, social and family adjustment and subtyping. In: Barkley RA, editor. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York: The Guilford Press; 2006: 184-218.
- Caron C, Rutter M. Comorbidity in child psychopathology: concepts, issues and research strategies. J Child Psychol Psychiatry 1991;32:1063-80.
- Venkatesh C, Ravikumar T, Andal A, Virudhagirinathan BS. Attention- deficit / hyperactivity disorder in children: clinical profile and co- morbidity. Indian J Psychol Med 2012;34:34-8.
- Palaniappan P, Seshadri S, Girimaji SC, Srinath S. Pattern of comorbidities in Indian children and adolescents with attention deficit hyperactivity disorder. Eur Psychiatry 2013;28(Suppl 1):1.
- Srinivasaraghavan R, Mahadevan S, Kattimani S. Impact of comorbidity on three month follow-up outcome of children with ADHD in a child guidance clinic: preliminary report. Indian J Psychol Med 2013;35:346-51.
- Chess S, Thomas A. Temperament: theory and practice. New York: Brunner / Mazel; 1996.
- DuPaul GJ, Power TJ, Anastopulos AD, Reid R. ADHD Rating Scale IV: Checklist, norms, and clinical interpretation. New York, NY: The Guilford Press; 1998.
- Sheehan DV, Sheehan KH, Shytle RD, Janavs J, Bannon Y, Rogers JE, et al. Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J Clin Psychiatry 2010;71:313-26.
- Guy W, editor. ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976.
- Shaffer D, Gould M, Brasic J, Ambrosini P, Fisher P, Bird H, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry 1983;40:1228-31.
- Malin AJ. Vineland Social Maturity Scale — Nagpur adaptation. Lucknow, India: Indian Psychological Corporation; 1971.
- Malin AJ. Malin’s intelligence scale for Indian children. Nagpur, India: Child Guidance Centre; 1969.
- Schopler E, Reichler RJ, Rochen Renner B. The Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1988.
- Kapur M, John A, Rozario J, Oommen A. NIMHANS Index of specific learning disabilities 1991. In: Hirisave U, Oommen A, Kapur M, editors. Psychological assessment of children in the clinical setting. Bangalore: National Institute of Mental Health and Neuro Sciences; 2006: 72-121.
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, et al. Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. J Am Acad Child Adolesc Psychiatry 2002;41:262-8.
- Amiri S, Shafiee-Kandjani AR, Fakhari A, Abdi S, Golmirzaei J, Akbari Rafi Z, et al. Psychiatric comorbidities in ADHD children: an Iranian study among primary school students. Arch Iran Med 2013;16:513-7.
- Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry 1999;40:57-87.
- Lakhan R. The coexistence of psychiatric disorders and intellectual disability in children aged 3-18 years in the Barwani district, India. ISRN Psychiatry 2013;2013:875873.
- Chung SY, Luk SL, Lee PW. A follow-up study of infantile autism in Hong Kong. J Autism Dev Disord 1990;20:221-32.
- Tannock R. Rethinking ADHD and LD in DSM-5: proposed changes in diagnostic criteria. J Learn Disabil 2013;46:5-25.
- Yuce M, Zoroglu SS, Ceylan MF, Kandemir H, Karabekiroglu K. Psychiatric comorbidity distribution and diversities in children and adolescents with attention deficit / hyperactivity disorder: a study from Turkey. Neuropsychiatr Dis Treat 2013;9:1791-9.
- Tannock R. Attention deficit / hyperactivity disorder with anxiety disorders. In: Brown TE, editor. Attention-deficit disorders and comorbidities in children, adolescents and adults. Washington, DC: American Psychiatric Press; 2000: 125-70.
- Moderators and mediators of treatment response for children with attention-deficit / hyperactivity disorder: the Multimodal Treatment Study of Children with Attention-Deficit / Hyperactivity Disorder. Arch Gen Psychiatry 1999;56:1088-96.
- Barkley RA. Associated cognitive, developmental, and health problems. In: Barkley RA, editor. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. 3rd ed. New York: The Guilford Press; 2006: 122-83.
- Gillberg C, Gillberg IC, Rasmussen P, Kadesjo B, Soderstrom H, Rastam M, et al. Co-existing disorders in ADHD — implications for diagnosis and intervention. Eur Child Adolesc Psychiatry 2004;13(1 Suppl):i80-92.